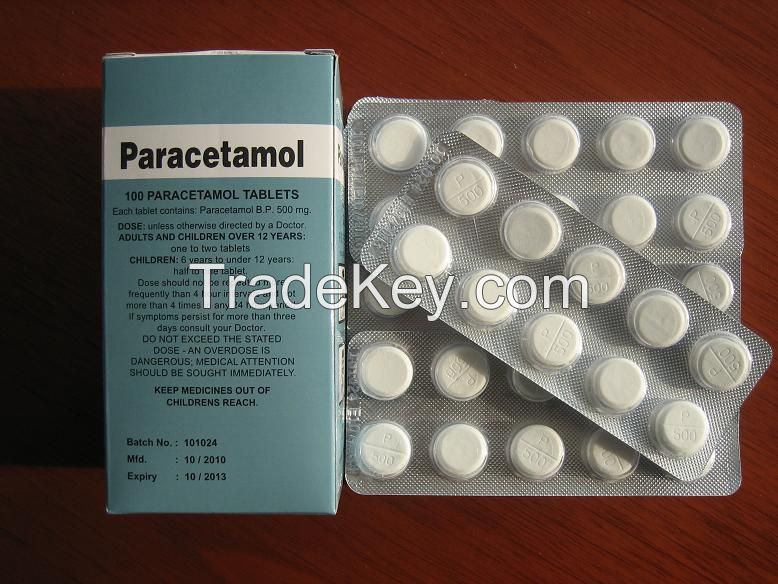
Le paracétamol Comprimés 500 mg / 325mg BPF La FDA a approuvé le paracétamol antipyrétique - Chine Le paracétamol, l'Acétaminophène
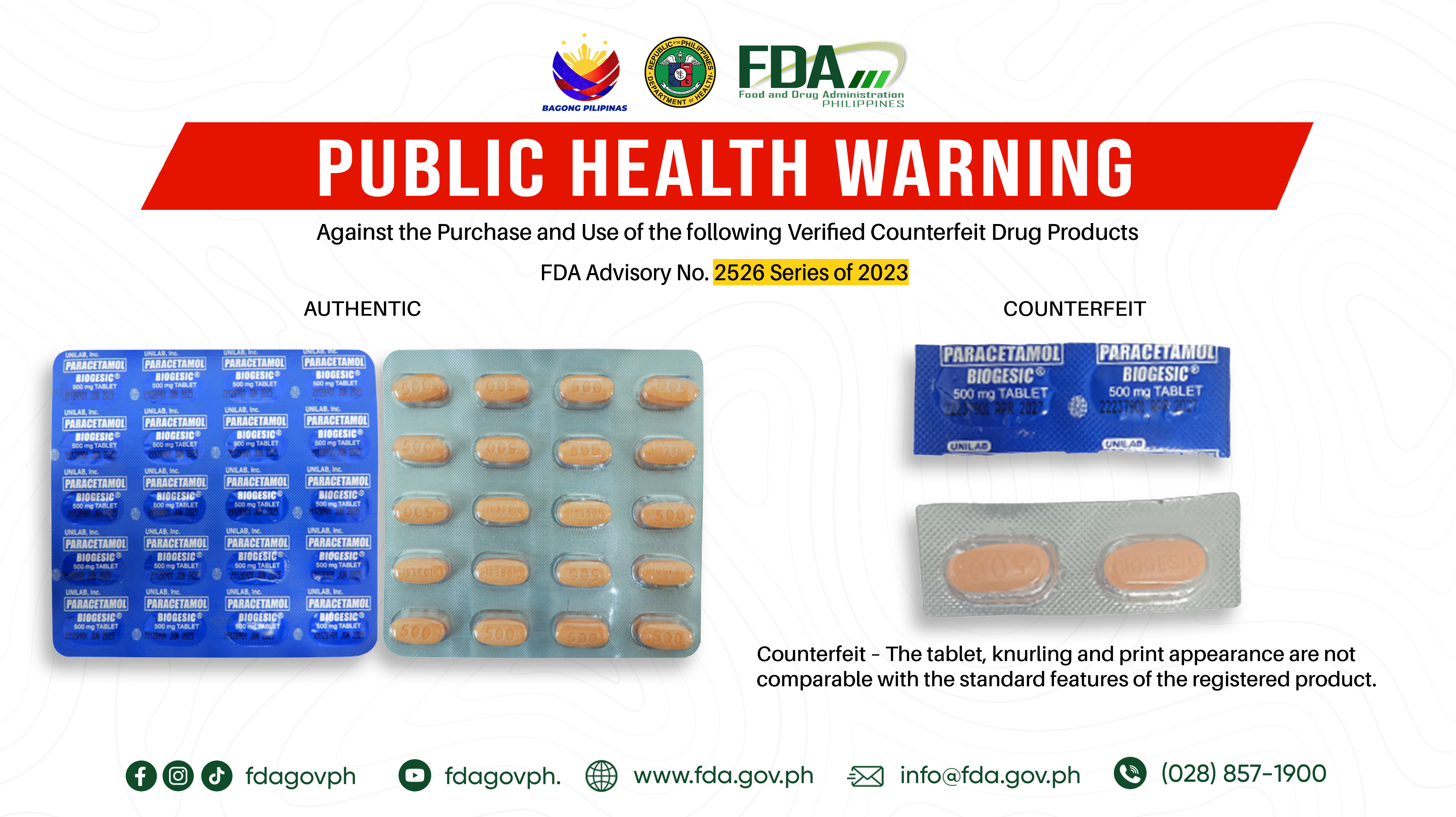
FDA Advisory No.2023-2526 || Public Health Warning Against the Purchase and Use of the following Verified Counterfeit Drug Products: - Food and Drug Administration

FDA Advisory No.2022-0939 || Public Health Warning Against the Purchase and Use of the following Verified Counterfeit Drug Products: - Food and Drug Administration

FDA Advisory No.2021-1612 || Public Health Warning Against the Purchase and Use of the Unregistered Drug Product “Paracetamol Tablets USP 100mg” - Food and Drug Administration

FDA Advisory No.2022-0134 || Public Health Warning Against the Purchase and Use of the Verified Counterfeit Drug Product “Phenylephrine HCl/ Chlorphenamine Maleate/ Paracetamol (Bioflu®) 10 mg/ 2 mg/ 500 mg Film-Coated Tablet” -

Les comprimés de paracétamol BPF La FDA a approuvé l'allégement 350mg - Chine Le paracétamol, l'Acétaminophène

FDA Advisory No.2022-0939 || Public Health Warning Against the Purchase and Use of the following Verified Counterfeit Drug Products: - Food and Drug Administration
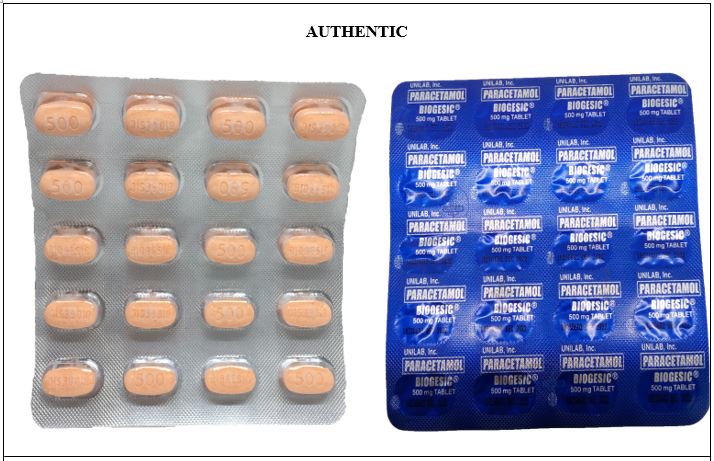
FDA Advisory No. 2020-1348 || Public Health Warning Against the Purchase and Use of the following Counterfeit Drug Products: - Food and Drug Administration
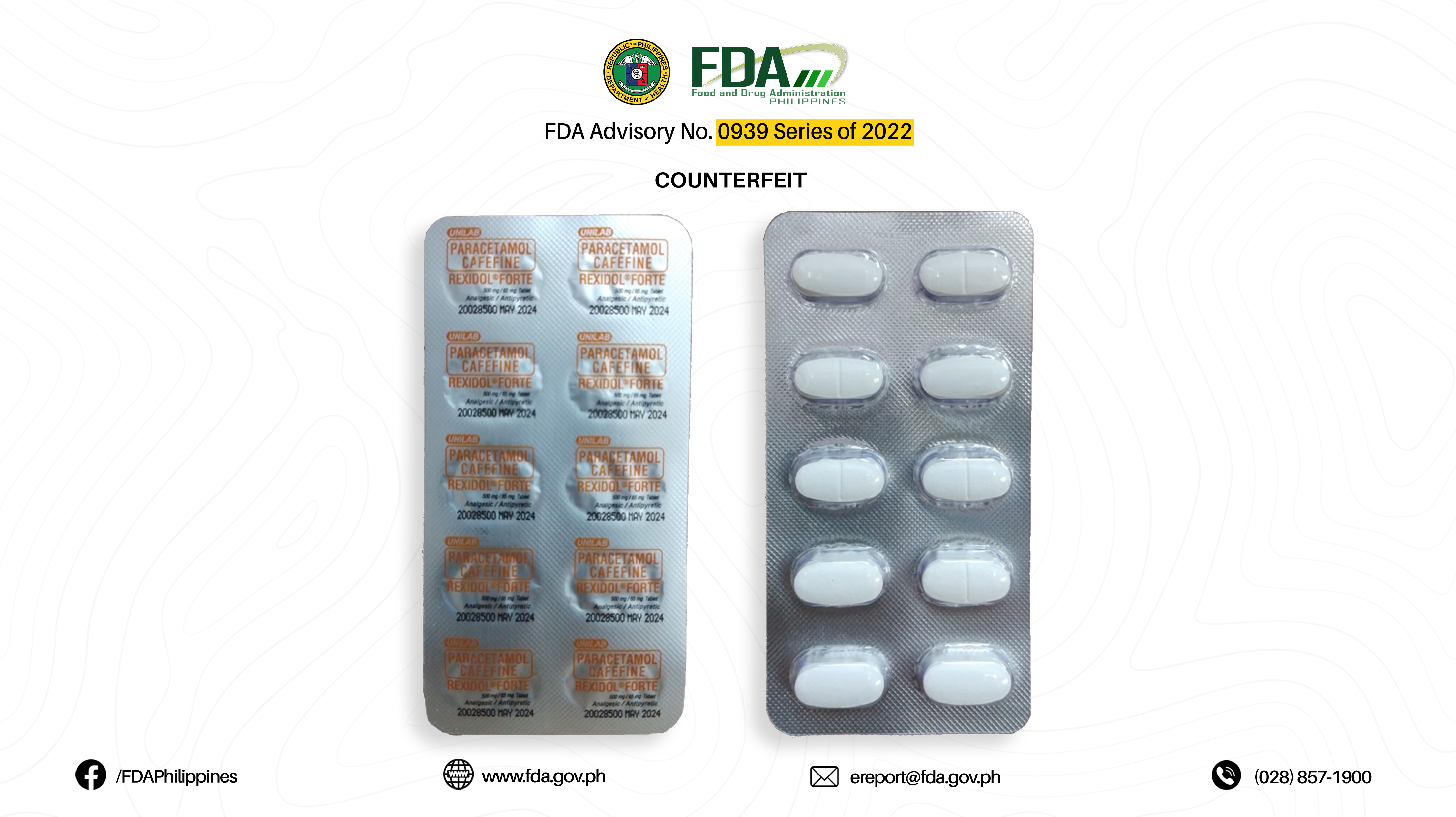
FDA Advisory No.2022-0939 || Public Health Warning Against the Purchase and Use of the following Verified Counterfeit Drug Products: - Food and Drug Administration
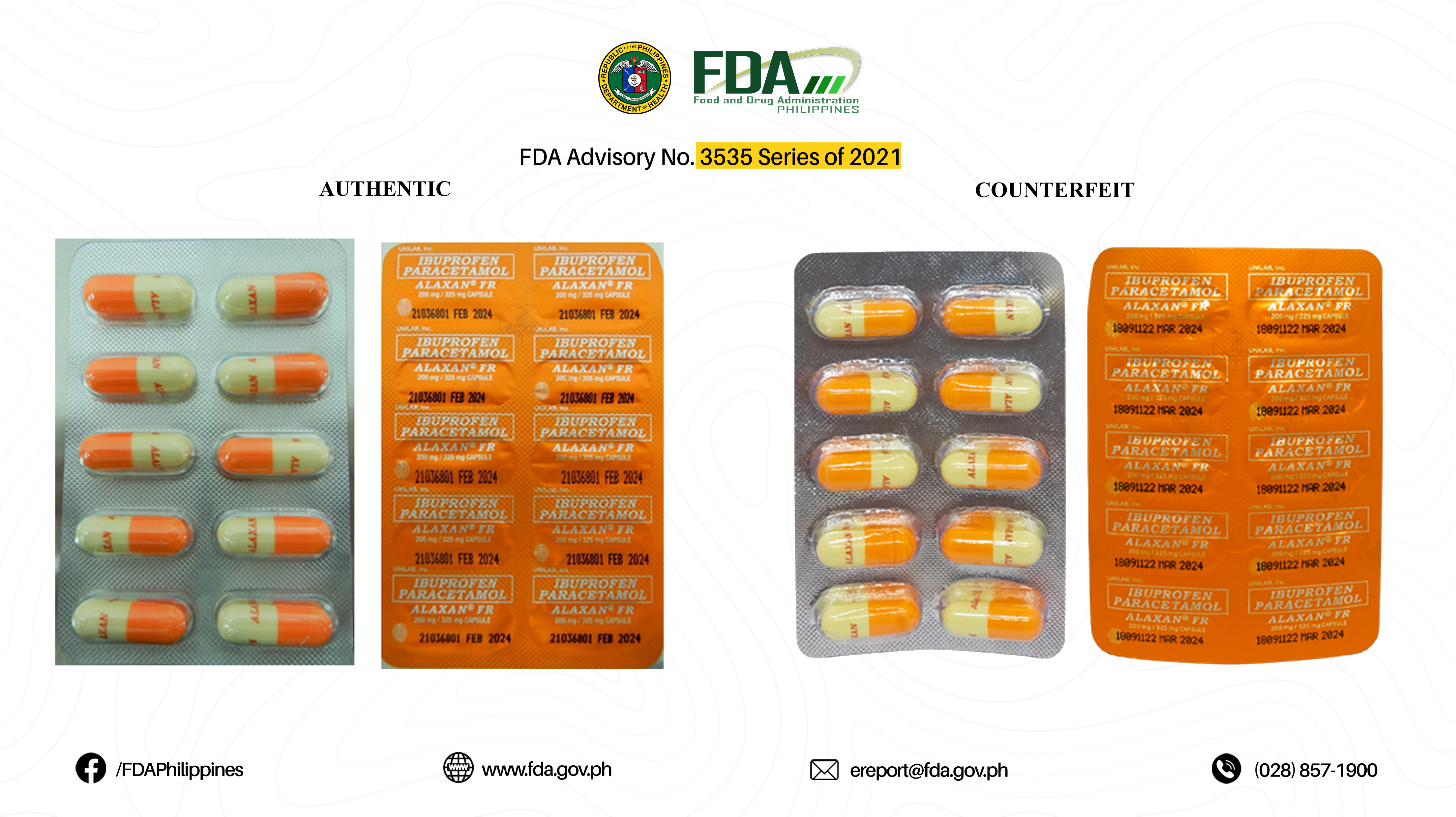
FDA Advisory No.2021-3535 || Public Health Warning Against the Purchase and Use of the following Verified Counterfeit Drug Products: - Food and Drug Administration

FDA Advisory No.2021-1723 || Public Health Warning Against the Purchase and Use of the Unregistered Drug Product “Perfalgan® Paracetamol 1 g in 100 mL Solution for Infusion 100 mL Vial” - Food and Drug Administration

FDA Advisory No.2022-0779 || Public Health Warning Against the Purchase and Use of the following Verified Counterfeit Drug Products: - Food and Drug Administration

FDA Advisory No.2022-0008 || Public Health Warning Against the Purchase and Use of the Verified Counterfeit Drug Product “Phenylpropanolamine HCl/ Chlorphenamine Maleate/ Paracetamol (Decolgen® Forte) 25 mg/ 2 mg/ 500 mg Tablet” -

GMP FDA Approved Drugs Paracetamol Injection for Reducing Fever and Pain by Dawa Pharmaceutical Co.,Ltd, Made in China

FDA Advisory No.2022-0277 || Public Health Warning Against the Purchase and Use of the Verified Counterfeit Drug Product : - Food and Drug Administration

FDA Advisory No.2022-0939 || Public Health Warning Against the Purchase and Use of the following Verified Counterfeit Drug Products: - Food and Drug Administration

FDA Advisory No.2023-1840 || Public Health Warning on Substandard (Contaminated) Paracetamol + Phenylephrine Chlorhydrate + Chlorpheniramine Maleate Syrup Confirmed by the World Health Organization (WHO) - Food and Drug Administration