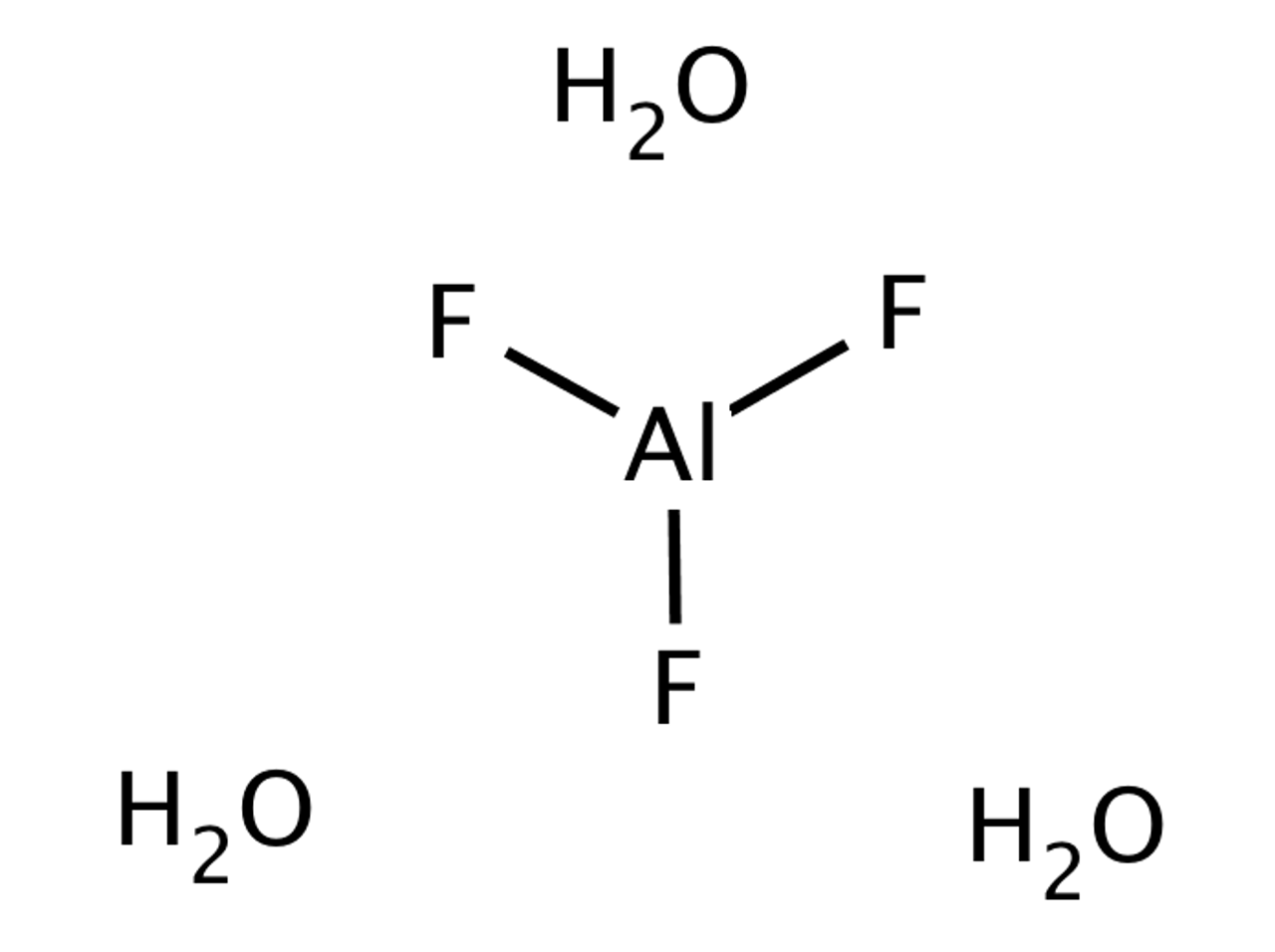
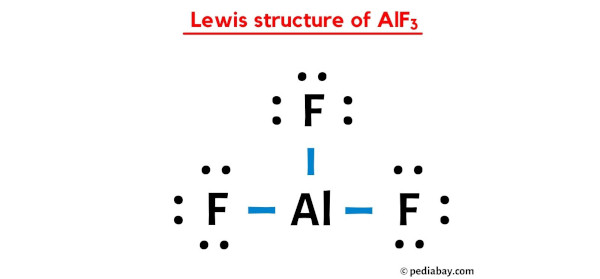
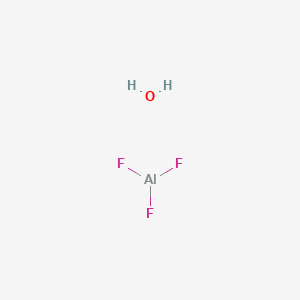
![PDF] STABILITY RELATIONS OF ALUMINUM HYDROXY-FLUORIDE HYDRATE, A RALSTONITE-LIKE MINERAL, IN THE SYSTEM AlF3–Al2O3–H2O–HF | Semantic Scholar PDF] STABILITY RELATIONS OF ALUMINUM HYDROXY-FLUORIDE HYDRATE, A RALSTONITE-LIKE MINERAL, IN THE SYSTEM AlF3–Al2O3–H2O–HF | Semantic Scholar](https://d3i71xaburhd42.cloudfront.net/6ecc39c373f3ffa84ca5f44bf21fda67029c3564/8-Figure4-1.png)
PDF] STABILITY RELATIONS OF ALUMINUM HYDROXY-FLUORIDE HYDRATE, A RALSTONITE-LIKE MINERAL, IN THE SYSTEM AlF3–Al2O3–H2O–HF | Semantic Scholar

Possible Formation of H3O+ Cations Due to Aluminum Fluoride Interactions with Water. | Semantic Scholar

PDF) Stability of the AlF3 surface in H2O and HF environments: An investigation using hybrid density functional theory and atomistic thermodynamics | Sven Schroeder - Academia.edu
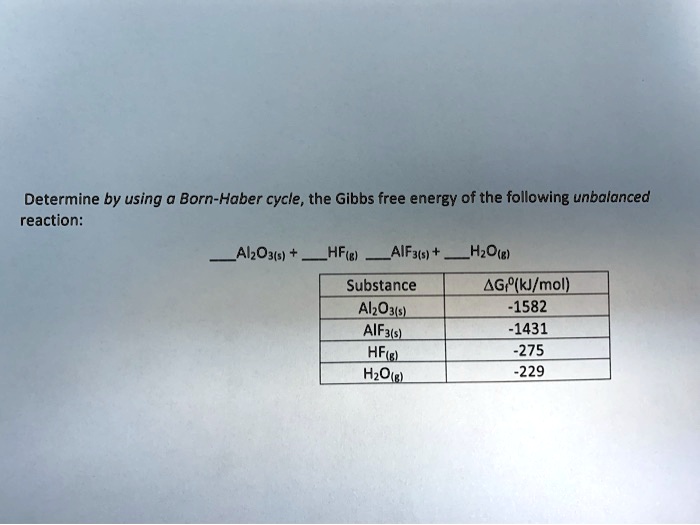
SOLVED: Determine, by using the Born-Haber cycle, the Gibbs free energy of the following unbalanced reaction: Al2O3(s) + 2HF(g) â†' 2AlF3(s) + 3H2O(l) AGr%(kJ/mol) -1582 1431 -275 -229 Substance Al2O3(s) AlF3(s) HF(g)

Hydrate de fluorure d'aluminium, Puratronic , 99,99 % (base métallique), Thermo Scientific Chemicals | Fisher Scientific

Atomic Layer Deposition of AlF3 Using Trimethylaluminum and Hydrogen Fluoride | The Journal of Physical Chemistry C

The acid strength of the datively bound complexes involving AlF3 lone pair acceptor and various lone pair donors - ScienceDirect
![PDF] Competition between Al2O3 atomic layer etching and AlF3 atomic layer deposition using sequential exposures of trimethylaluminum and hydrogen fluoride. | Semantic Scholar PDF] Competition between Al2O3 atomic layer etching and AlF3 atomic layer deposition using sequential exposures of trimethylaluminum and hydrogen fluoride. | Semantic Scholar](https://d3i71xaburhd42.cloudfront.net/e2ac2e88133be5a7278abdd433a3dd75d426a246/2-Figure1-1.png)
PDF] Competition between Al2O3 atomic layer etching and AlF3 atomic layer deposition using sequential exposures of trimethylaluminum and hydrogen fluoride. | Semantic Scholar
![The missing hydrate AlF3·6H2O [Al(H2O)6]F3: Ionothermal synthesis, crystal structure and characterization of aluminum fluoride hexahydrate - ScienceDirect The missing hydrate AlF3·6H2O [Al(H2O)6]F3: Ionothermal synthesis, crystal structure and characterization of aluminum fluoride hexahydrate - ScienceDirect](https://ars.els-cdn.com/content/image/1-s2.0-S1293255816303363-fx1.jpg)
The missing hydrate AlF3·6H2O [Al(H2O)6]F3: Ionothermal synthesis, crystal structure and characterization of aluminum fluoride hexahydrate - ScienceDirect

The molecular graphs of the AlF3-C2H2 (the top left), AlBr3-C2H4 (the... | Download Scientific Diagram

The isomeric structures of the HClO4/n(AlF3) superacids (for n = 1–2).... | Download Scientific Diagram