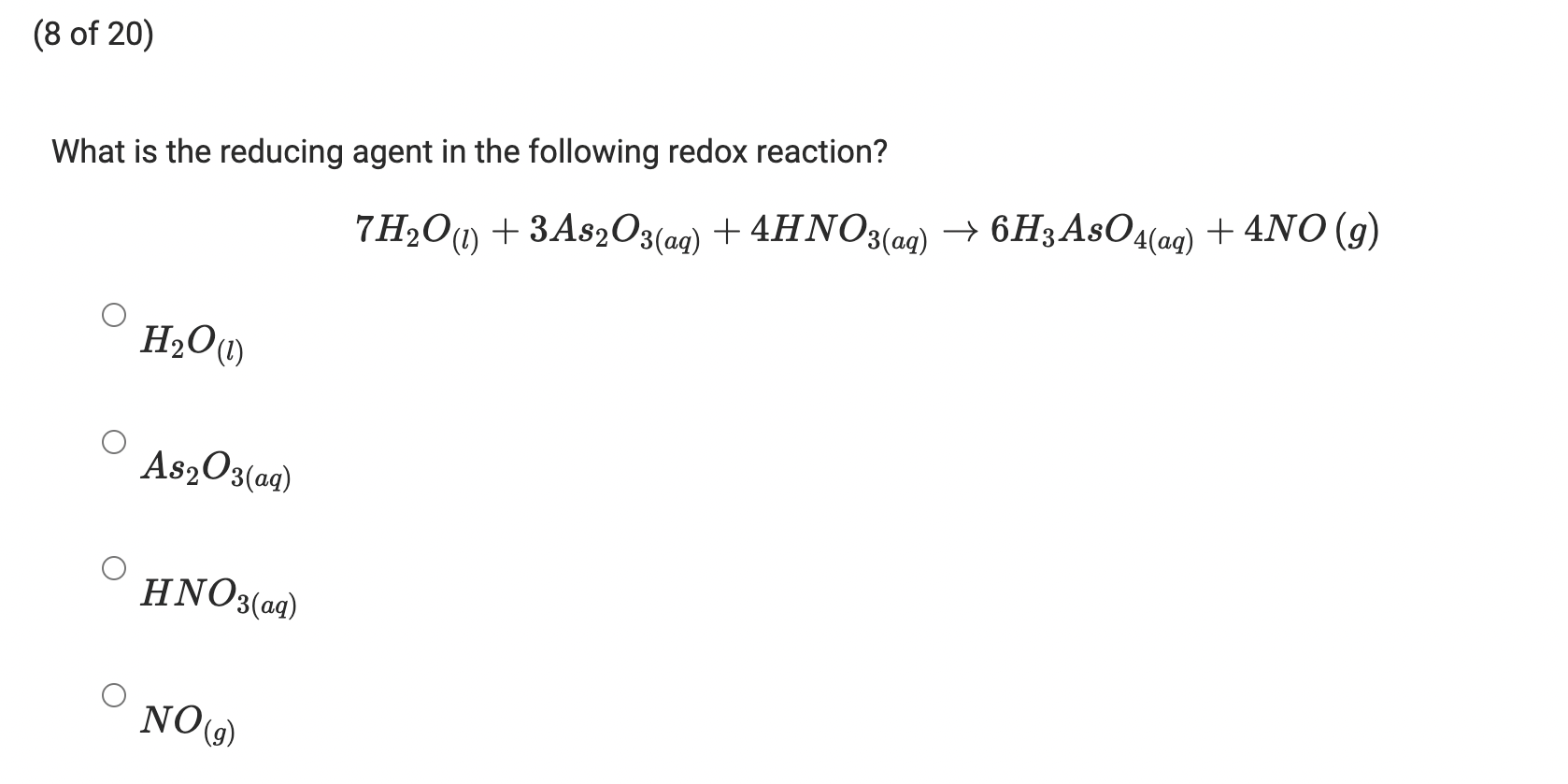SOLVED: A solution of iodine was standardized by titration with H3AsO3 prepared from weighing and dissolving 141.6 mg of pure As2O3. The titration reaction is H3AsO3 + I2 + H2O = H3AsO4 +
Może ktoś rozwiązać redoxa, robiąc to bilansem elektronowo-jonowym?As2O3 + HNO3 + H2O -> H3AsO4 + N2O3 - Brainly.pl
24. In the following reaction(unbalanced) n factor of As2S3 is As2S3 + H+ + NO3 1 NO + H2O + AsO4 3 + S04 2 (2) 4 (3) 24 (4) 28
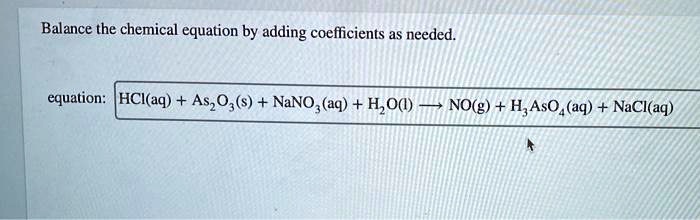
SOLVED: Balance the chemical equation by adding coefficients as needed. equation: HCl(aq) + As2O3(s) + NaNO3(aq) + H2O(l) â†' NO(g) + H3AsO4(aq) + NaCl(aq)
Balance the following equation using oxidation number method AS2S3 + HNO3 + H2O → H3ASO4 + H2SO4 + NO - Sarthaks eConnect | Largest Online Education Community
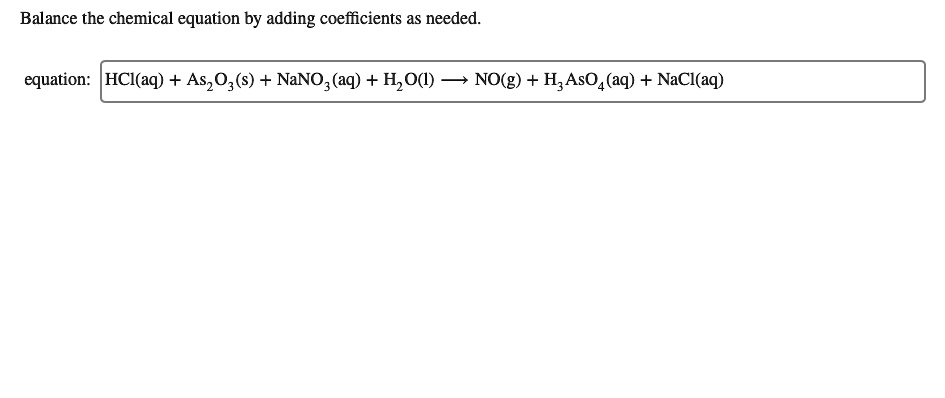
SOLVED: Balance the chemical equation by adding coefficients as needed. Equation: HCl(aq) + As2O3(s) + NaNO2(aq) + H2O(l) â†' NO(g) + H3AsO4(aq) + NaCl(aq)

SOLVED: When this equation is properly balanced, the coefficients of As2O3 and H2O are, respectively, As2O3 + H2O â†' HAsO3 + ? 0l4 and ? 0l2 and ? Oland 2 Ol3ald 1







![ANSWERED] A primary standard grade As2O3 M M 197 84... - Physical Chemistry - Kunduz ANSWERED] A primary standard grade As2O3 M M 197 84... - Physical Chemistry - Kunduz](https://media.kunduz.com/media/sug-question-candidate/20210920005325449887-2402955.jpg)