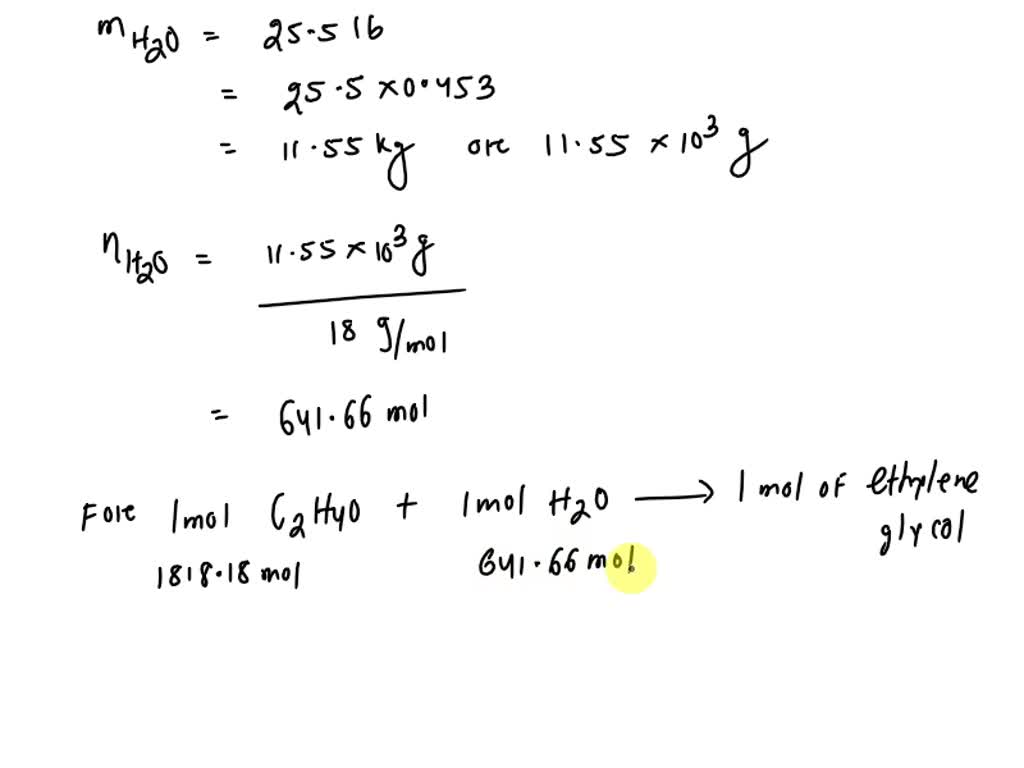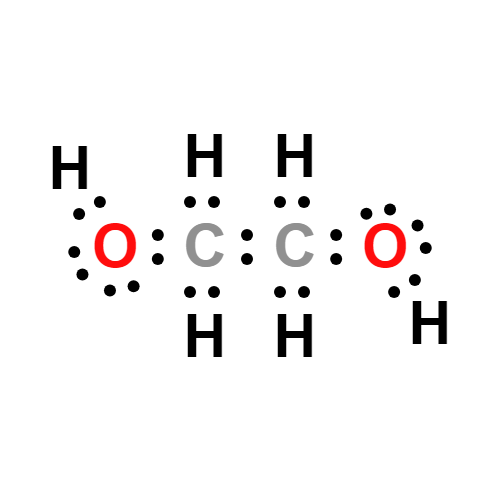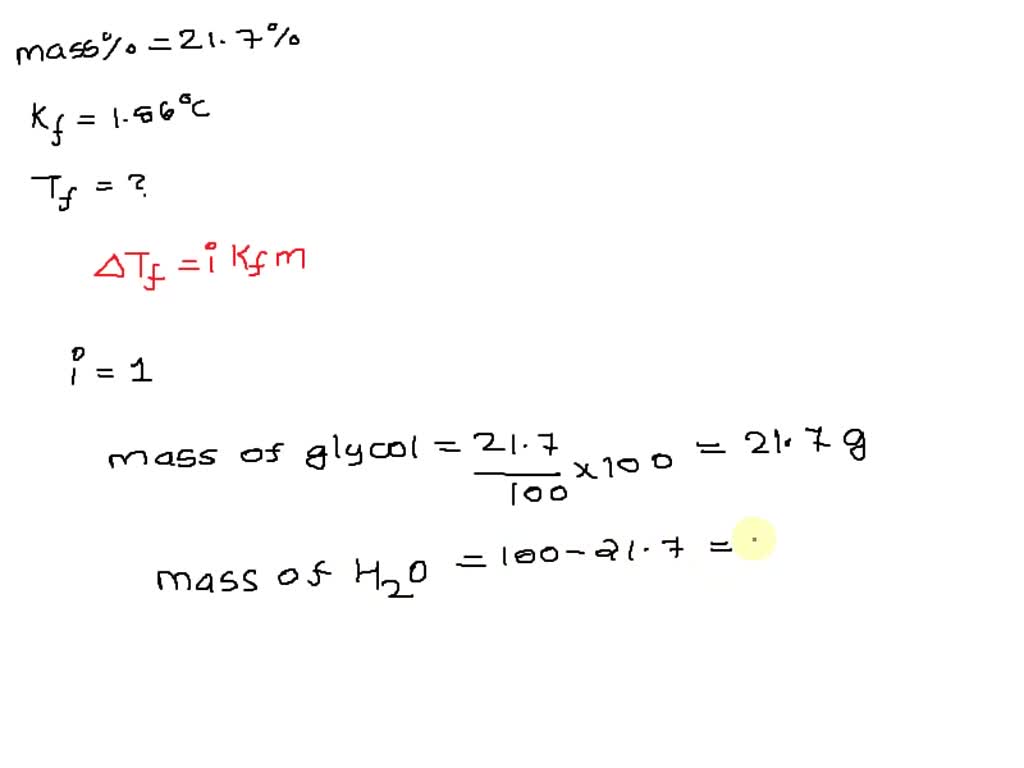
SOLVED: C2H6O2 is infinitely miscible (soluble) in water. Ethylene Glycol is a nonelectrolyte that is used as antifreeze. What is the lowest possible melting point for engine coolant that is 21.7% (by
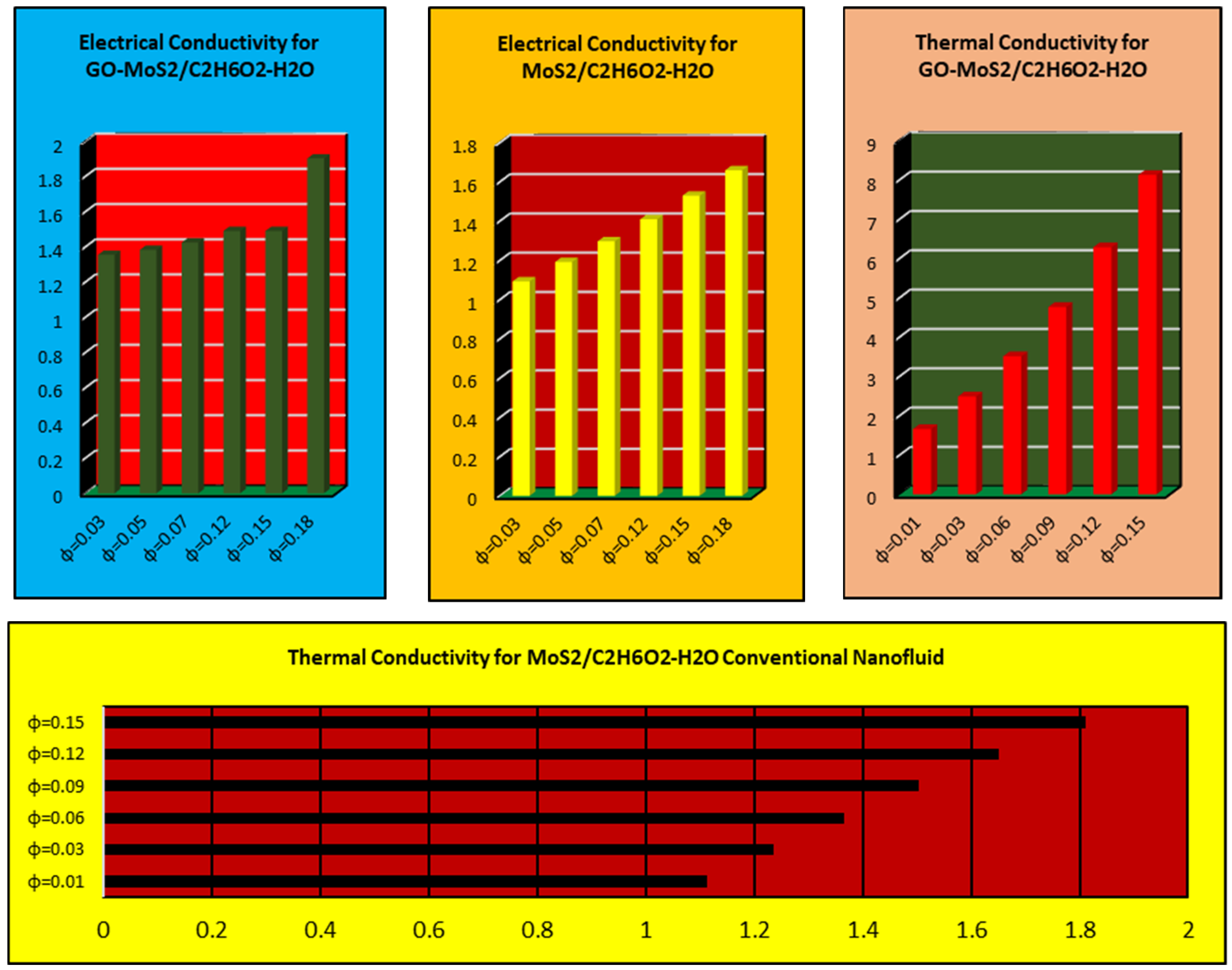
Molecules | Free Full-Text | Thermal Transport Investigation in Magneto-Radiative GO-MoS2/H2O-C2H6O2 Hybrid Nanofluid Subject to Cattaneo–Christov Model
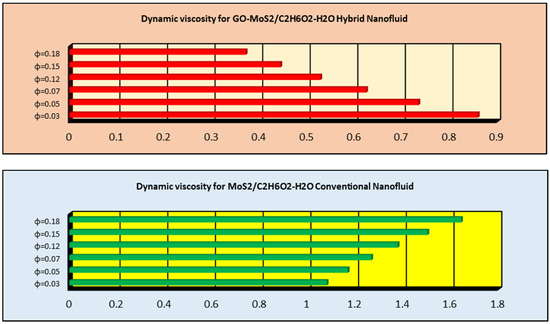
Molecules | Free Full-Text | Thermal Transport Investigation in Magneto-Radiative GO-MoS2/H2O-C2H6O2 Hybrid Nanofluid Subject to Cattaneo–Christov Model

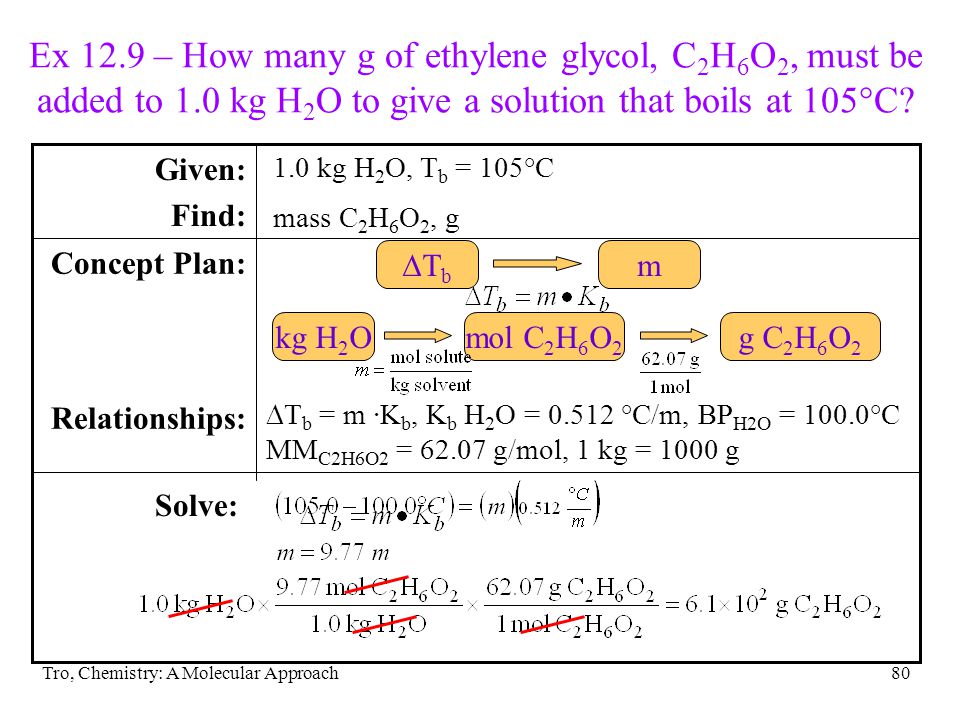
SOLVED: What is the freezing point of a solution prepared from 53.3 g ethylene glycol (C2H6O2) and 86.4 g H2O? Kf of water is 1.86 °C/m. a. -28.7 b. -18.5 c. -14.3 d. -14.0 e. -22.9
![PDF] Second Law Analysis of Magneto Radiative GO-MoS2/H2O–(CH2OH)2 Hybrid Nanofluid | Semantic Scholar PDF] Second Law Analysis of Magneto Radiative GO-MoS2/H2O–(CH2OH)2 Hybrid Nanofluid | Semantic Scholar](https://d3i71xaburhd42.cloudfront.net/17b308d5b8da1ef74c61af055385521209c6dd97/15-Figure13-1.png)
PDF] Second Law Analysis of Magneto Radiative GO-MoS2/H2O–(CH2OH)2 Hybrid Nanofluid | Semantic Scholar

Numerical thermal study on CNTs/ C2H6O2– H2O hybrid base nanofluid upon a porous stretching cylinder under impact of magnetic source - ScienceDirect
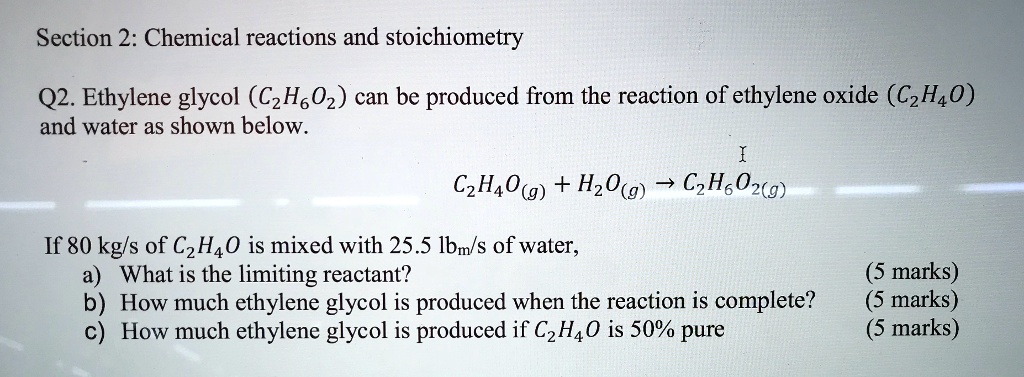
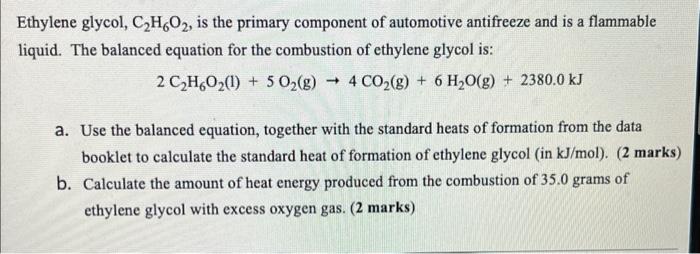
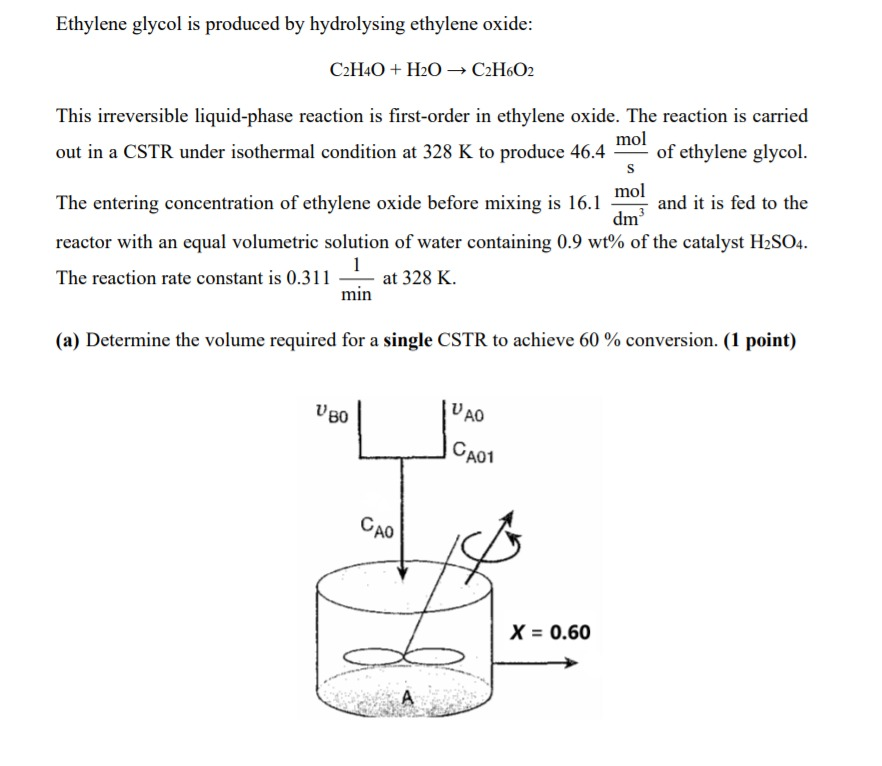
SOLVED: Section 2: Chemical reactions and stoichiometry Q2. Ethylene glycol (C2H6O2) can be produced from the reaction of ethylene oxide (C2H4O) and water as shown below: C2H4O(g) + H2O(g) -> C2H6O2(g) If

Impact of Q for Fe3O4−C2H6O2−H2O and Fe3O4−Co/C2H6O2−H2O on fη and θη... | Download Scientific Diagram

Full article: Numerical study for temperature-dependent viscosity based unsteady flow of GP-MoS2/C2H6O2-H2O over a porous stretching sheet

Impact of Q for Fe3O4−C2H6O2−H2O and Fe3O4−Co/C2H6O2−H2O on fη and θη... | Download Scientific Diagram

What is ethylene glycol in organic chemistry? | by KAKALI GHOSH , Teacher,blogger. M.Sc chemistry. | Medium