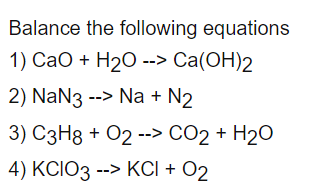1. The reaction between CaO and H2O is a) highly exothermic with hissing b) endothermic with hissing sound - Brainly.in

The mineralogy of the CaO–Al2O3–SiO2–H2O (CASH) hydroceramic system from 200 to 350 °C - ScienceDirect
![Structure of the polymeric [Ca(H2O)6]2+ center in hydrated calcium chloride, illustrating the high coordination number typical for calcium complexes. : r/dankmemes Structure of the polymeric [Ca(H2O)6]2+ center in hydrated calcium chloride, illustrating the high coordination number typical for calcium complexes. : r/dankmemes](https://i.redd.it/kodg7rcdf3q11.jpg)
Structure of the polymeric [Ca(H2O)6]2+ center in hydrated calcium chloride, illustrating the high coordination number typical for calcium complexes. : r/dankmemes

Please help with all parts and show work. The decomposition of Ca(OH)_2(s) into CaO(s) and H_2O(g) at constant pressure requires the addition of 109 kj of heat per mole of Ca(OH)_2. (a)

If 32.5 grams of CaO are dissolved in 212 grams of water, what is the concentration of the solution in percent by mass? | Socratic
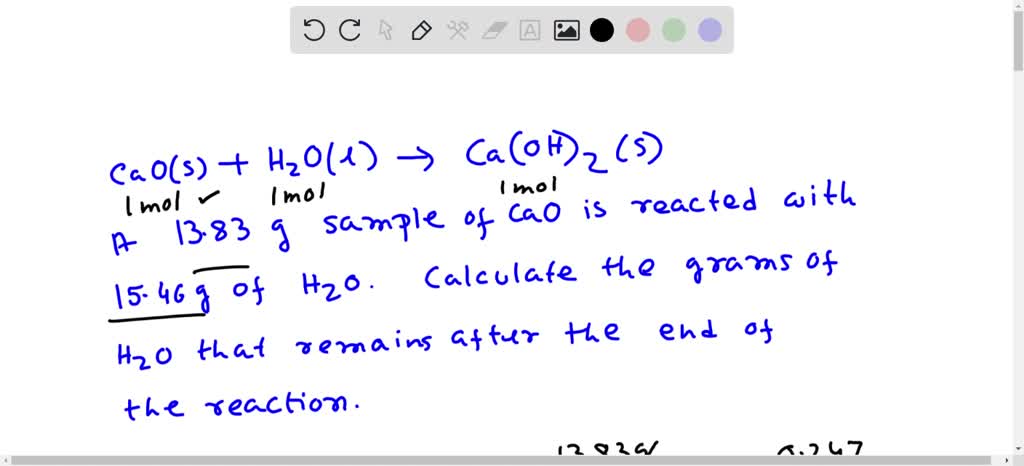
SOLVED: Calcium oxide reacts with water in a combination reaction to produce calcium hydroxide: CaO(s) + H2O(l) → Ca(OH)2 (s) A 13.83 g sample of CaO is reacted with 15.46 g of

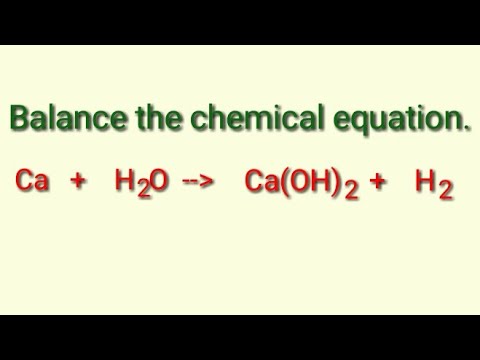
How to Balance Ca + H2O = Ca(OH)2 + H2 (Calcium plus Water) | How to Balance Ca + H2O = Ca(OH)2 + H2 (Calcium plus Water) Hi Guys, welcome back to

Insight into the Mechanism and Effect of H2O on CaO Sulfation by Density Functional Theory | Energy & Fuels

Are you confused with Uncertainty and Uncertainty? CaO -neglected lime (calcium oxide) Ca(OH)2.. | VK