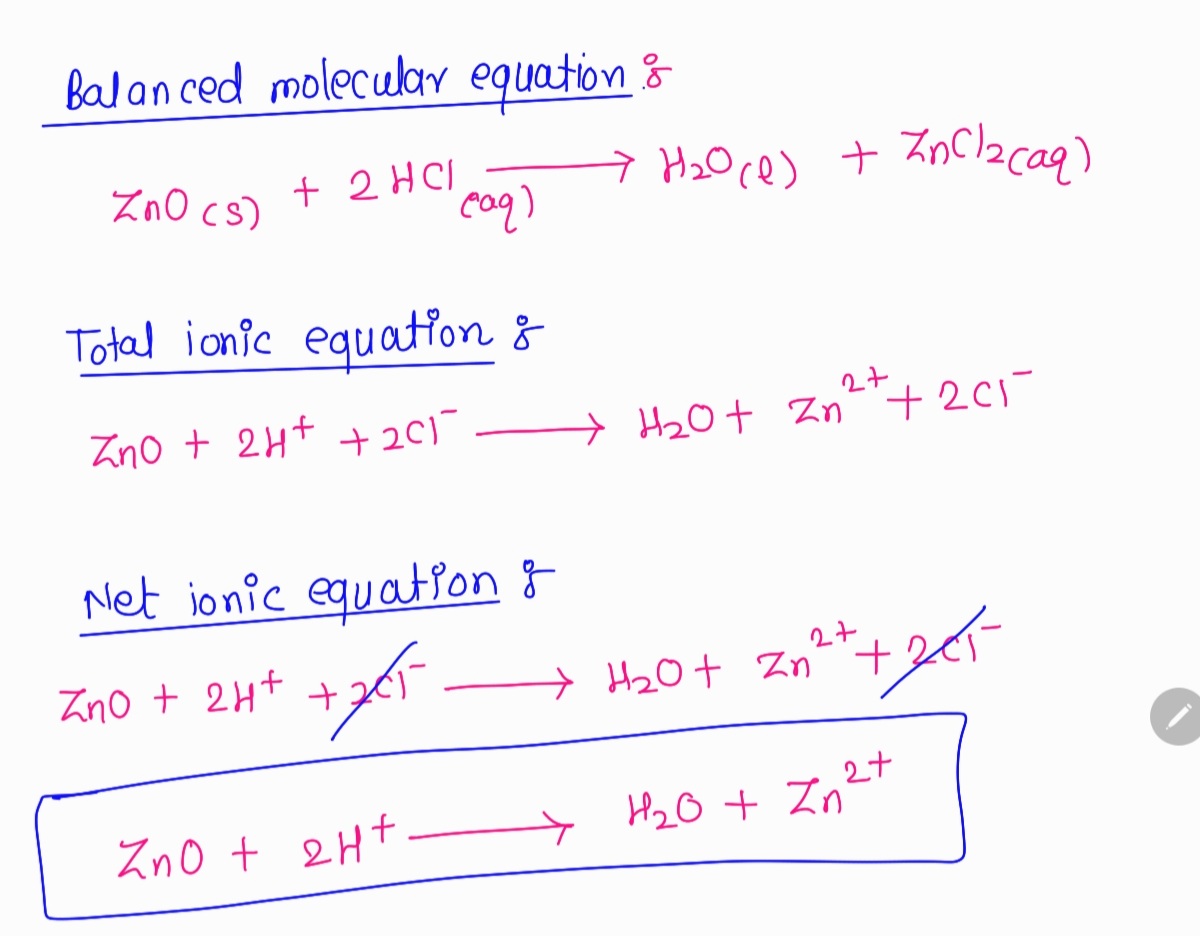
Self-Supplying of Hydrogen Peroxide/Oxygen Based on CaO2-Co3O4 Cascade Nanoreactors for Cellular Microenvironment Regulation | ACS Applied Nano Materials

C) Cal24 Pulice (d) All of these - Cl2 2 → A - Auto-oxidation Ca(OH)2 -H20 → CaCl2 + B Dry cao2 Identify B in the above reaction :

Steady release-activation of hydrogen peroxide and molecular oxygen towards the removal of ciprofloxacin in the FeOCl/CaO2 system - ScienceDirect

SOLVED: Consider the combustion of Ca. What are the expected products of the reaction? Select all that apply: CaO2 CaZo H2O CaO CO2

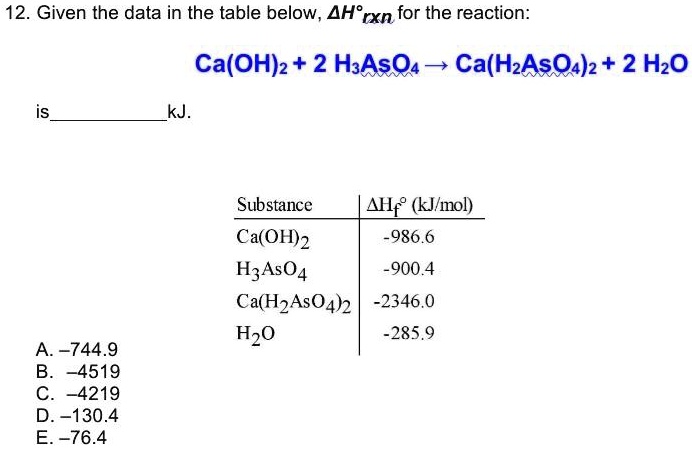
Please help with all parts and show work. The decomposition of Ca(OH)_2(s) into CaO(s) and H_2O(g) at constant pressure requires the addition of 109 kj of heat per mole of Ca(OH)_2. (a)

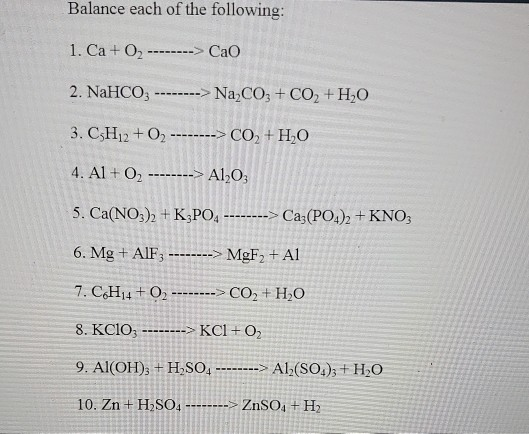
When heated, metal hydroxides decompose to produce a metal oxide and water. Selected the correct balanced - brainly.com

Influence of calcium peroxide on fermentation pattern and protozoa in the rumen: Archiv für Tierernaehrung: Vol 32, No 7-8

CaO2/UV Process for One-Step Phosphorus Removal and Recovery from Hypophosphite: Simultaneous Oxidation and Precipitation | ACS ES&T Water

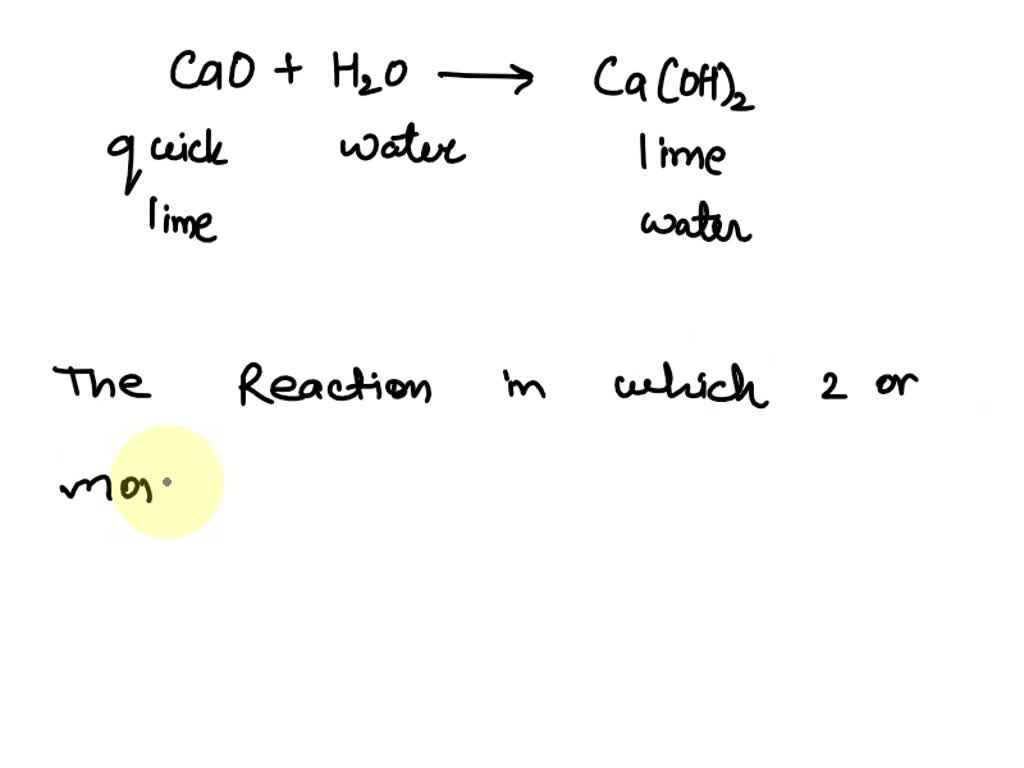


![Punjabi] What type of reactions are represented by following equation Punjabi] What type of reactions are represented by following equation](https://static.doubtnut.com/ss/web-overlay-thumb/10335489.webp)