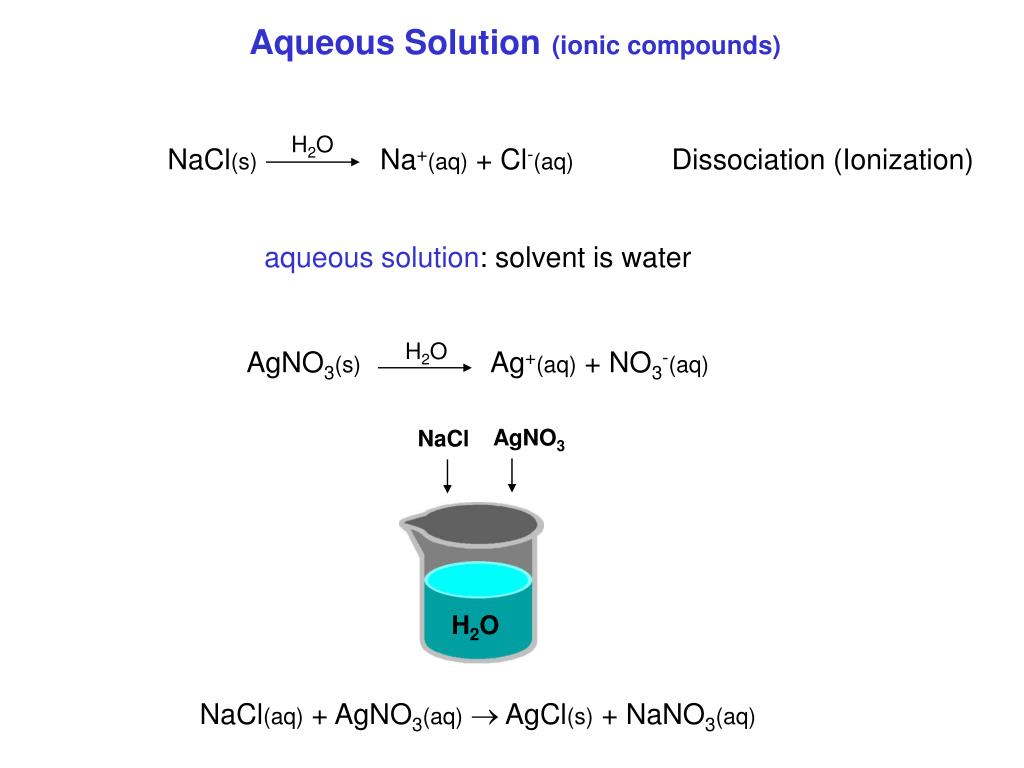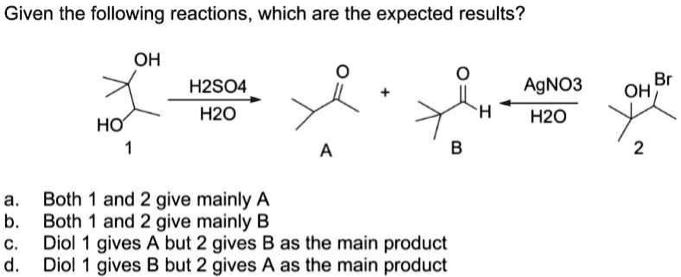
SOLVED: Given the following reactions, which are the expected results? OH H2SO4 H2O AgNO3 H2O OH Br HO Both 1 and 2 give mainly A. Both 1 and 2 give mainly B.

OneClass: Hess's Law (b) Given the following thermochemical data: Ag(s)+HNO3(aq)â†'AgNO3(aq)+½H2(g) ...
8. Assertion:Isobutaneiodidereacts with AgNO3/H2O to give tertbutylalcohol Reason:silver promoted hydride rearrangement direct the reaction

Intermolecular Oxidative Radical Addition to Aromatic Aldehydes: Direct Access to 1,4- and 1,5-Diketones via Silver-Catalyzed Ring-Opening Acylation of Cyclopropanols and Cyclobutanols | The Journal of Organic Chemistry
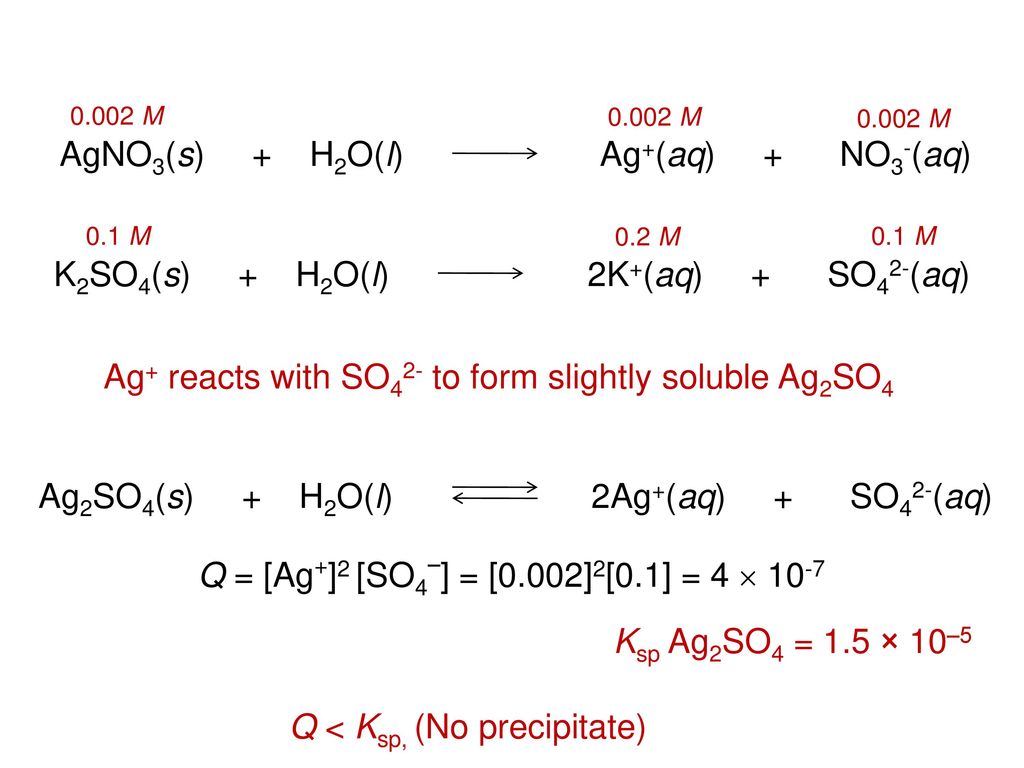
![The volume (in mL) of 0.1 M AgNO3 required for complete precipitation of chloride ions present in 30 mLof 0.01 M solution of [Cr(H2O)5Cl]Cl2, as silver chloride is close toCorrect answer is ' The volume (in mL) of 0.1 M AgNO3 required for complete precipitation of chloride ions present in 30 mLof 0.01 M solution of [Cr(H2O)5Cl]Cl2, as silver chloride is close toCorrect answer is '](https://edurev.gumlet.io/ApplicationImages/Temp/24d955be-fbfb-4c5b-9701-95bc154f720c_lg.jpg)