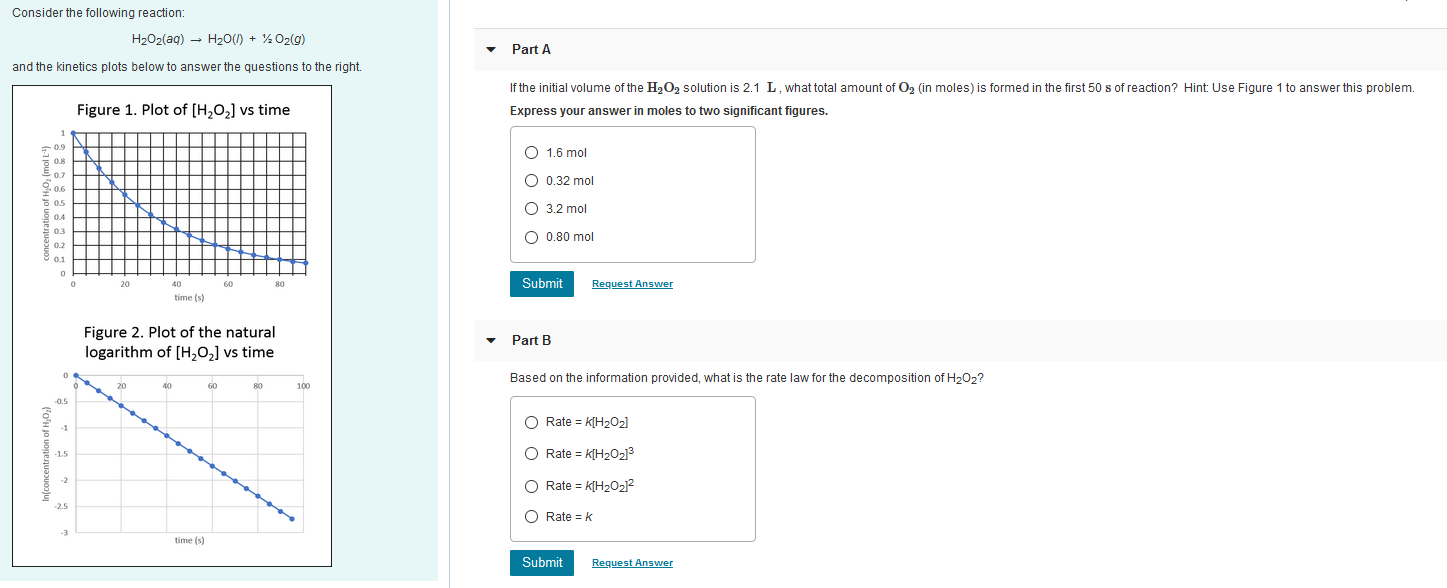
2H202 alkaline medium *2H20 + 02 the proposed mechanism is as given below : (1) H2O2 +1 → H2O+IO (slow) (2) H202 + 10 + H20+1+02 (fast) (i) Write rate law the

SOLVED: Consider the following chemical reaction mechanism: 1. H2O2 –> H2O + O (slow) 2. O + CF2Cl2. –> ClO + CF2Cl 3. ClO +O3 –> Cl + 2O2 4. Cl +

16.38d | Would hydrogen peroxide be a suitable candidate for fuels: H2O2(l) → H2O(g) + 1/2O2(g) - YouTube

Determine enthalpy of formation H2O2(1), using listed enthalpies of reaction: N2H4(1) + 2H2O2(1) → N2(g) + 4H2O(l); AH; =-818 kJ/mol N2H4(1) + O2(g) → N2(g) + 2H2O(1); 4.H; = -622 kJ/mol H2(g) +

SOLVED: Calculate ΔG° for the reaction H2O(g) + 1/2O2(g) = H2O2(g) at 603 K using the following data: H2(g) + O2(g) = H2O2(g) K = 1.21037 at 603 K 2H2(g) + O2(g) =
I) H2O2 + O3 → H2O + 2O2 (II) H2O2 + Ag2O → 2Ag + H2O + O2 Role of hydrogen peroxide in the - Sarthaks eConnect | Largest Online Education Community
![SOLVED: In the reaction H2O2(aq) â†' H2O(l) + 1/2 O2(g), the initial concentration of H2O2 is 0.2546 M, and the initial rate of reaction is 9.32×10^(-4) M/s. What will be [H2O2] at SOLVED: In the reaction H2O2(aq) â†' H2O(l) + 1/2 O2(g), the initial concentration of H2O2 is 0.2546 M, and the initial rate of reaction is 9.32×10^(-4) M/s. What will be [H2O2] at](https://cdn.numerade.com/ask_previews/68c50aa-0834-010e-1c31-cf1f024cbc64_large.jpg)
SOLVED: In the reaction H2O2(aq) â†' H2O(l) + 1/2 O2(g), the initial concentration of H2O2 is 0.2546 M, and the initial rate of reaction is 9.32×10^(-4) M/s. What will be [H2O2] at



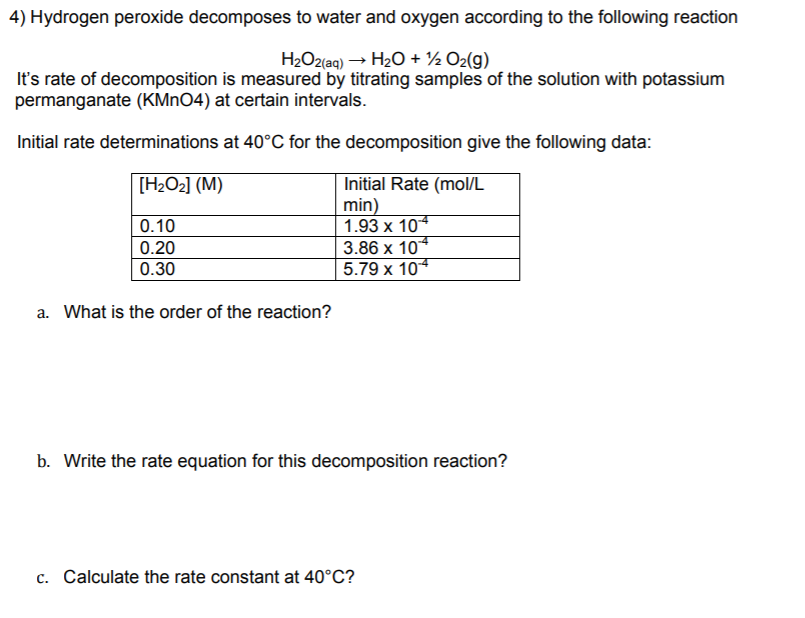
![Odia] H2O2 to H2O + 1/2 O2 is order reaction. Odia] H2O2 to H2O + 1/2 O2 is order reaction.](https://static.doubtnut.com/ss/web-overlay-thumb/11729098.webp)