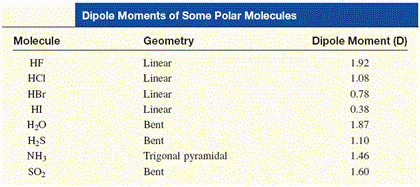
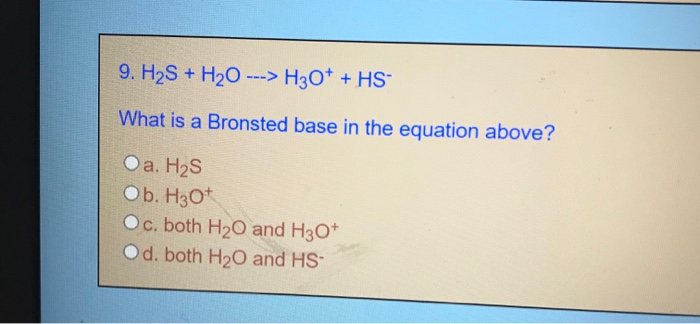
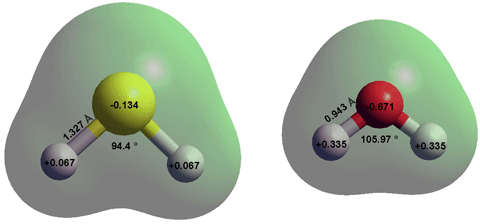
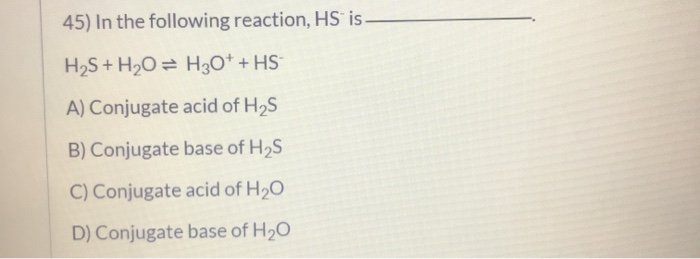
SOLVED: QUESTION 11 Is H2O or H2S more nucleophilic in protic solvent, and why? H2S is more nucleophilic because it is less solvated by the protic solvent: 0 H2O is more nucleophilic

How to Balance H2SO4 + HI = H2S + I2 + H2O We are back with another video wherein we help you balance the equation. It is a redox equation and for
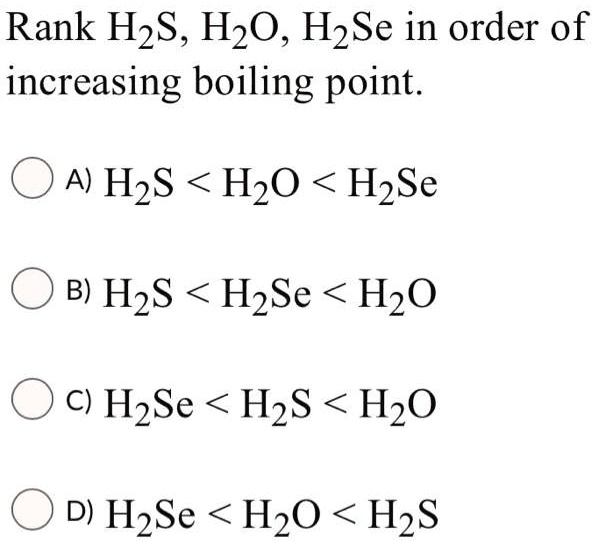
SOLVED: Rank H2S, H2O, H2Se in order of increasing boiling point. A) H2S < H2O < H2Se B) H2S < H2Se < H2O C) H2Se < H2S < H2O D) H2Se < H2O < H2S

Hydrogen Sulfide, Corrosion and BOD5 in Innovyze InfoSewer – ICM SWMM & ICM InfoWorks, SWMM5 & SWMM5+