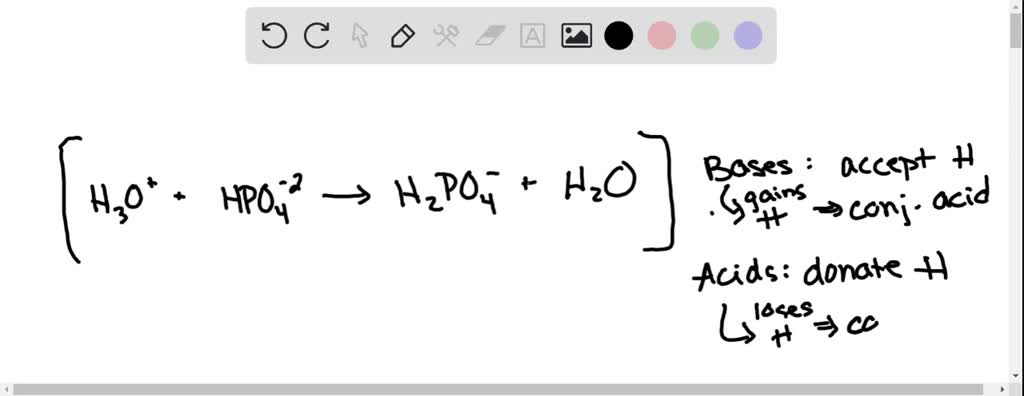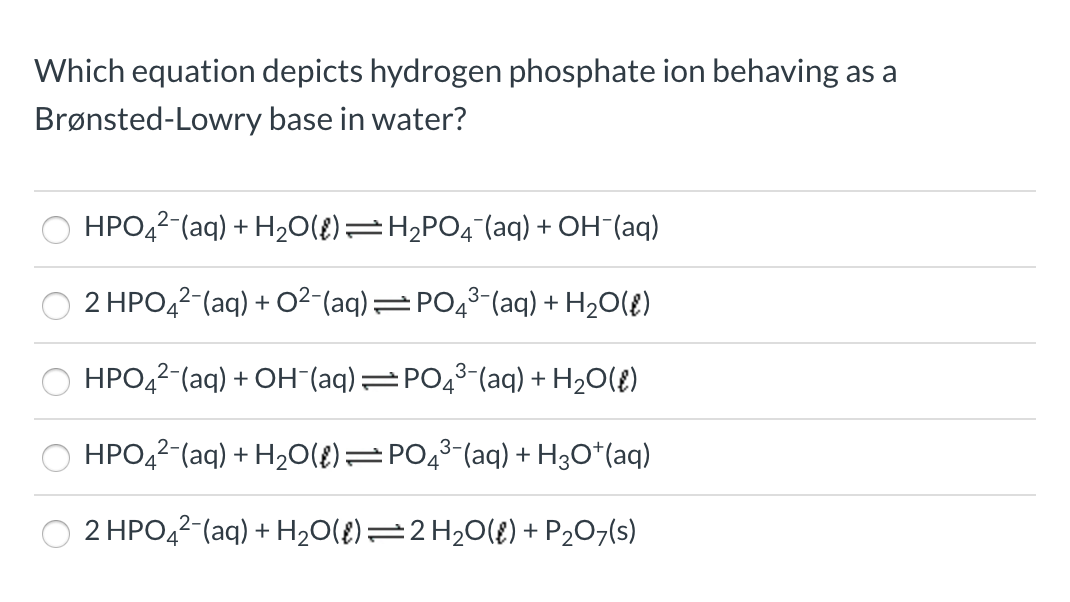![Na2[(VO)2(HPO4)2(C2O4)]·2H2O: A Promising Mixed Polyanionic Cathode Material for Aqueous Zn-Ion Batteries Na2[(VO)2(HPO4)2(C2O4)]·2H2O: A Promising Mixed Polyanionic Cathode Material for Aqueous Zn-Ion Batteries](https://pubs.acs.org/cms/10.1021/acs.inorgchem.2c03308/asset/images/medium/ic2c03308_0004.gif)
Na2[(VO)2(HPO4)2(C2O4)]·2H2O: A Promising Mixed Polyanionic Cathode Material for Aqueous Zn-Ion Batteries
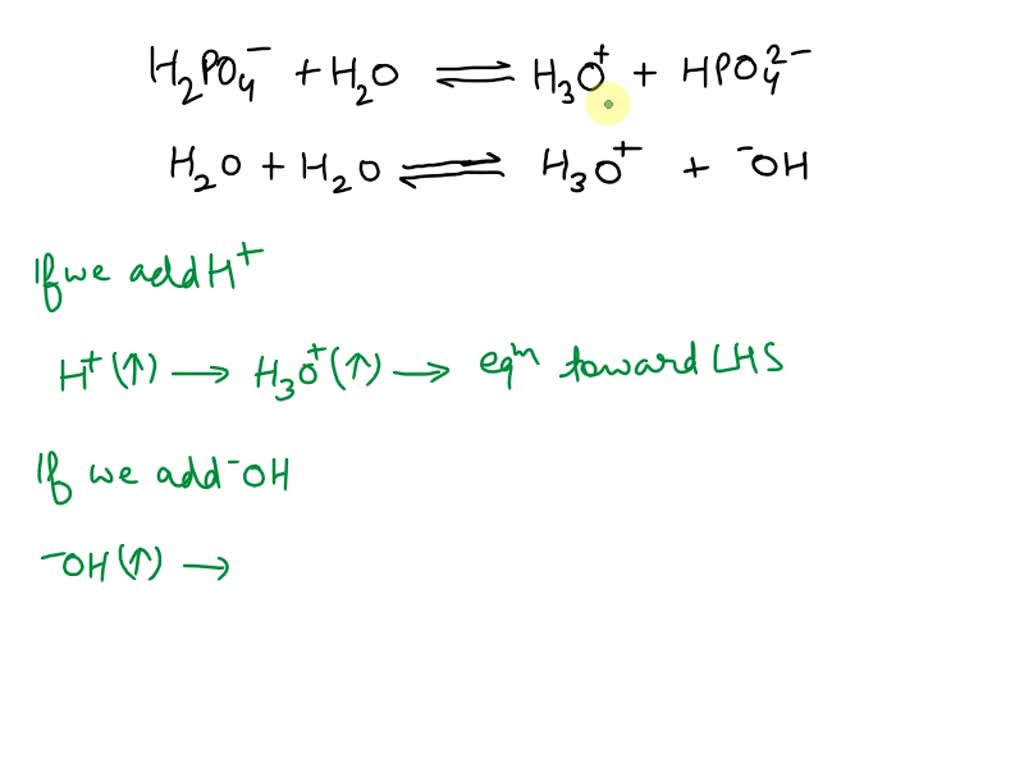
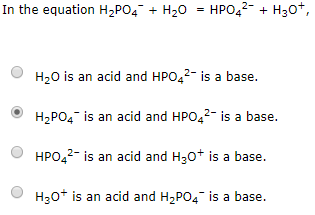
SOLVED: Relevant Equation H2PO4- + H2O <========> H3O+ + HPO4-2 Using Le Chatelier's Principle, explain why the phosphate buffer should have gone through a smaller change pH change compared to distilled water.

Figure 6 from VIIIVIV(HPO4)4·enH·H2O: a mixed-valence vanadium phosphate with an open framework | Semantic Scholar

Catalytic conversions of isocyanate to urea and glucose to levulinate esters over mesoporous α-Ti(HPO4)2·H2O in green media - X-MOL

Single-crystal neutron diffraction and Mössbauer spectroscopic study of hureaulite, (Mn,Fe)5(PO4)2 (HPO4)2 (H2O)4 - European Journal of Mineralogy Volume 28 Number 1 — Schweizerbart science publishers

PDF) Neutron powder diffraction study of α-Ti(HPO4)2.H2O and α-Hf(HPO4)2.H2O; H-atom positions | Pilar Pertierra - Academia.edu
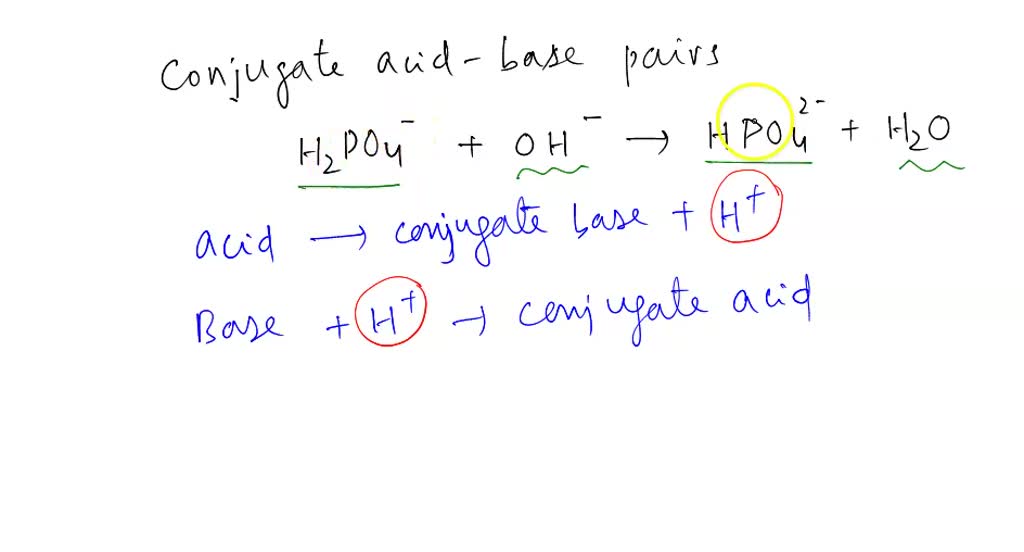
SOLVED: B. Identify the conjugate acid-base pairs in the following reactions: H2PO4- + OH- → HPO4-2 + H2O HBr + H2O → H3O+ + Br- CO3-2 + H2O → HCO3- + OH-

PDF) Thermal decomposition kinetics and reversible hydration study of the Li2Zn(HPO4)2�H2O | Chanaiporn Danvirutai - Academia.edu
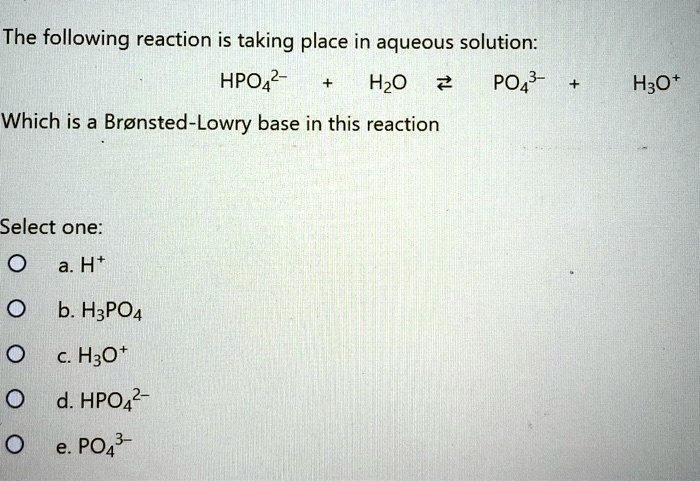
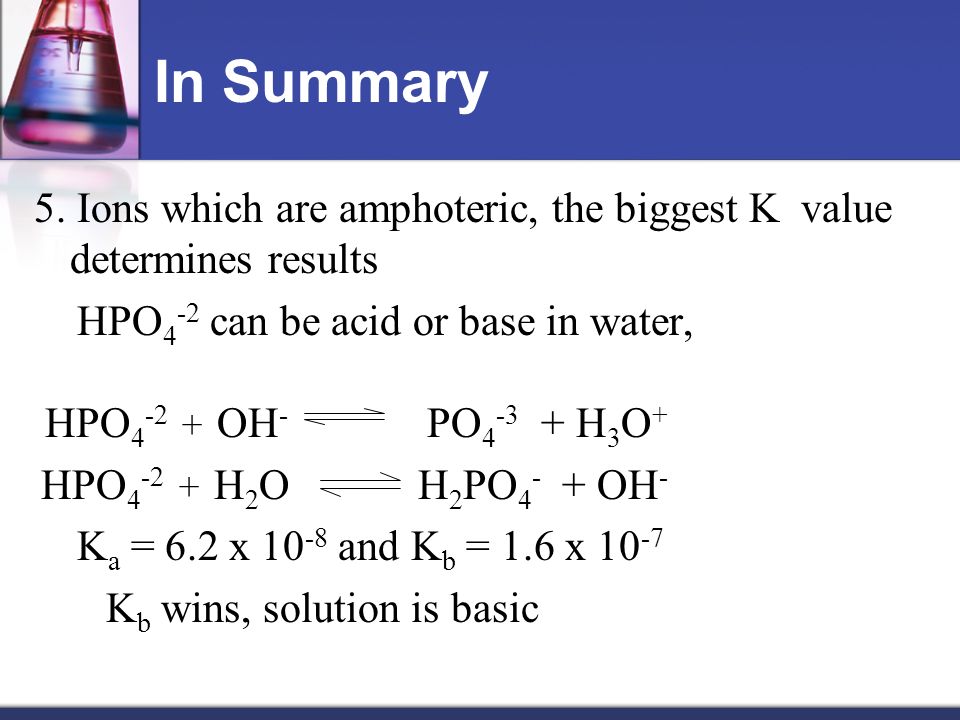
SOLVED: The following reaction is taking place in aqueous solution: HPO42- + H2O -> H3O+ + PO43-. Which is a Bronsted-Lowry base in this reaction? Select one: a. H+ b. H2PO4- c.

A new process for Na2Ca(HPO4)2 synthesis and its application as a heterogeneous catalyst in Knoevenagel condensation | Chehab | Mediterranean Journal of Chemistry
![Ca2[Ti(HPO4)2(PO4)]·H2O, Ca[Ti2(H2O)(HPO3)4]·H2O, and Ti(H2PO2)3: Solid-State Oxidation via Proton-Coupled Electron Transfer | Inorganic Chemistry Ca2[Ti(HPO4)2(PO4)]·H2O, Ca[Ti2(H2O)(HPO3)4]·H2O, and Ti(H2PO2)3: Solid-State Oxidation via Proton-Coupled Electron Transfer | Inorganic Chemistry](https://pubs.acs.org/cms/10.1021/acs.inorgchem.1c02685/asset/images/large/ic1c02685_0008.jpeg)