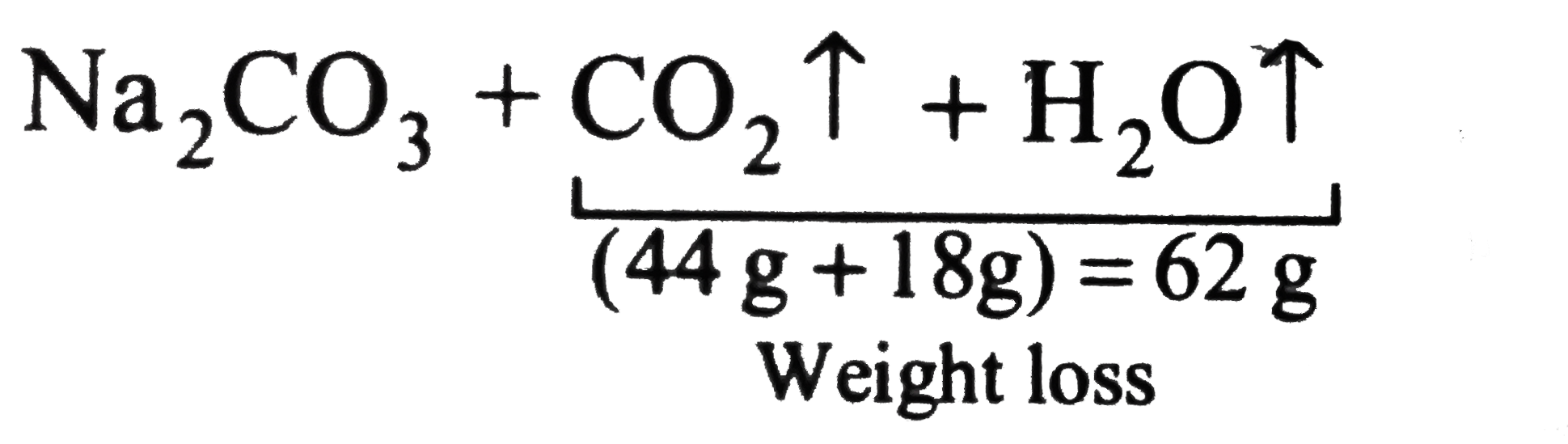How to Balance Na2CO3+H2O→NaOH+CO2 | How to Balance Na2CO3+H2O→NaOH+CO2 | By Chemistry 360 | Facebook
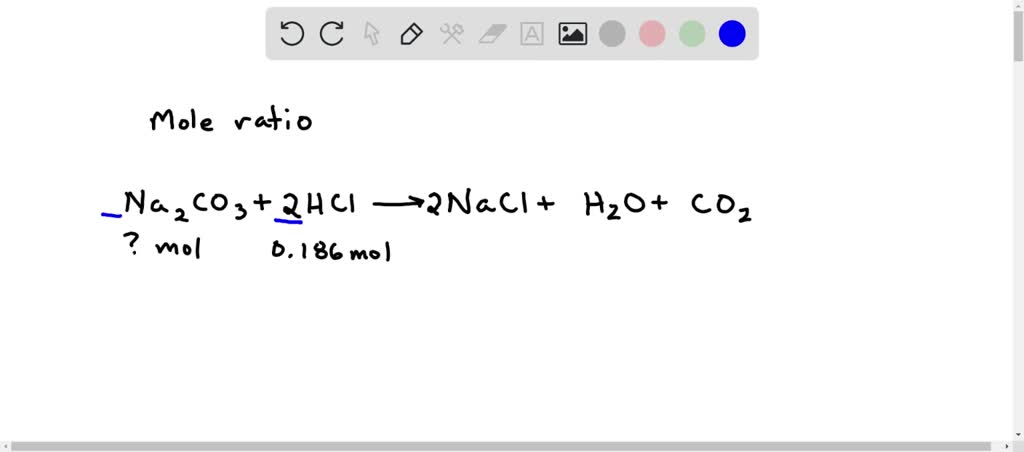
SOLVED: calculate the number of moles of sodium carbonate (Na2CO2) needed to neutralize 0,186 mole of HCI. chemical reaction : Na2CO3 + HCI —> NaCl + H20 + CO2
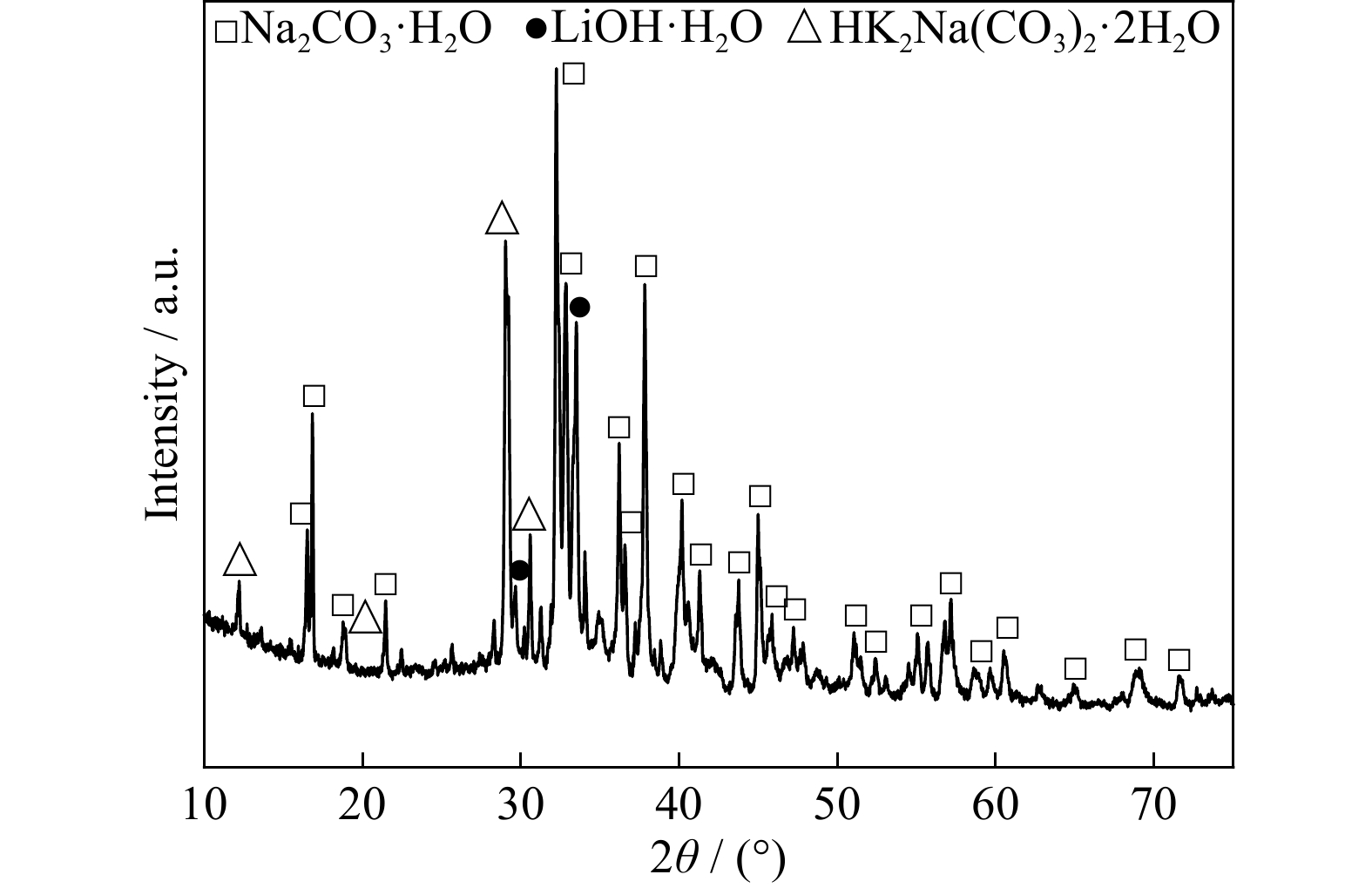
Extraction of lithium from the simulated pyrometallurgical slag of spent lithium-ion batteries by binary eutectic molten carbonates

Water content diagram of the quaternary system K + // H 2 PO 4 2− , SO... | Download Scientific Diagram

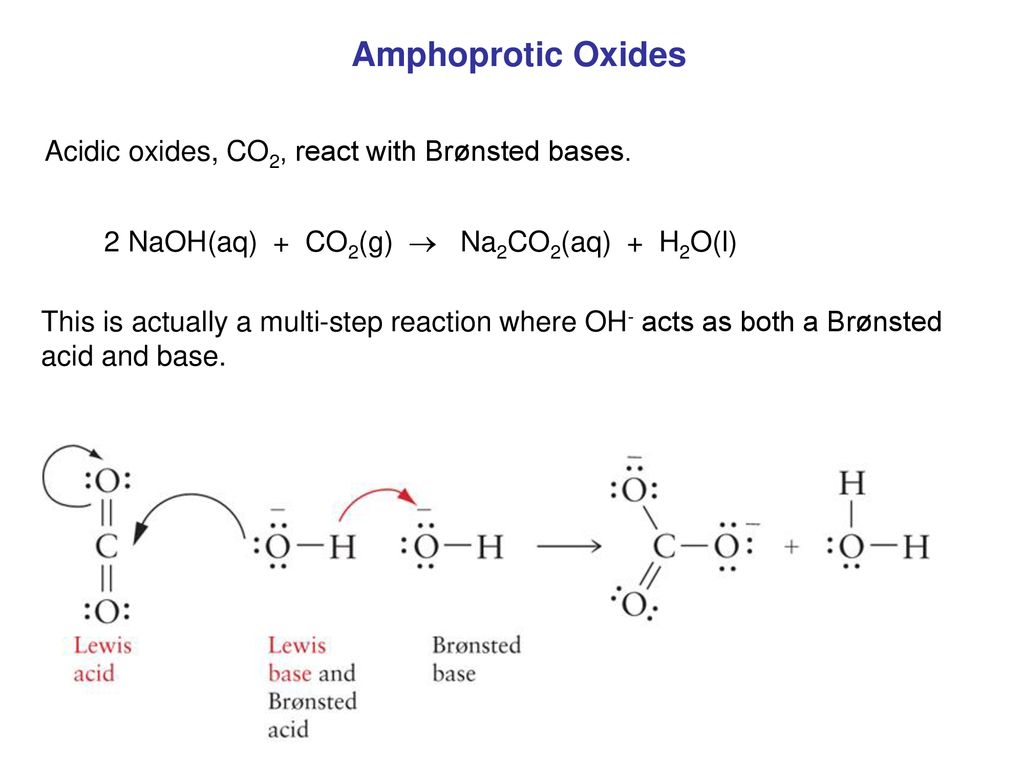
1 Dilute hydrochloric acid reacts with sodium carbonate solution. 2HCl(aq)+ Na2CO2(aq) - brainly.com

A Strategy for Amide to β-Oxo Ester Transformation via N-Alkenoxypyridinium Salts as the Activator and H2O as the Nucleophile | Organic Letters

Membrane Crystallization of Sodium Carbonate for Carbon Dioxide Recovery: Effect of Impurities on the Crystal Morphology | Crystal Growth & Design

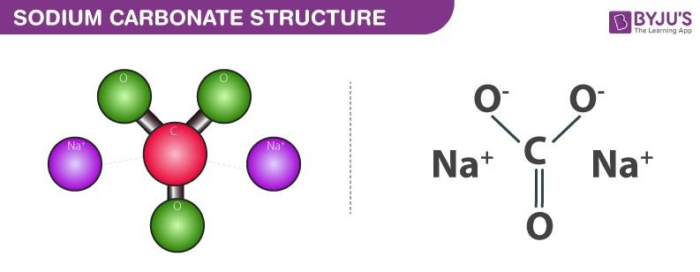
Sodium Carbonate (Na2CO3) Reaction: What does Sodium Carbonate do in a Reaction? - Aakash BYJU'S Blog
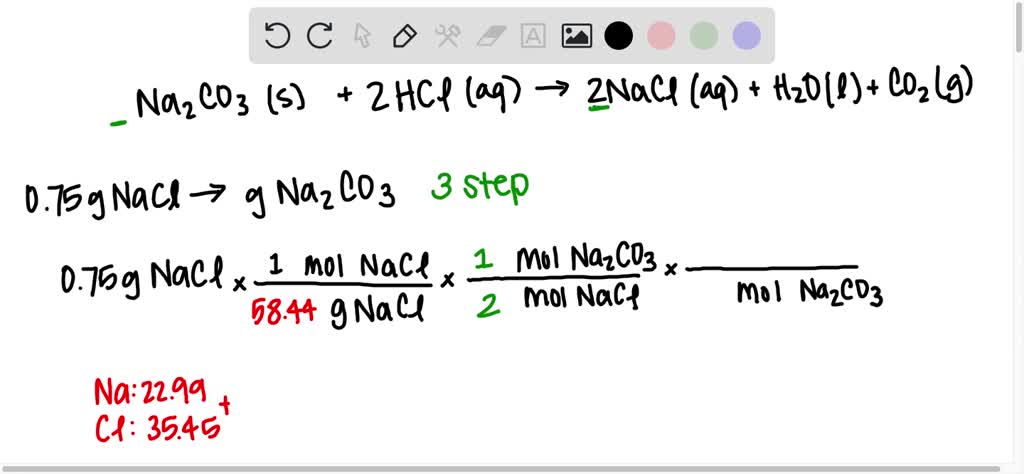
SOLVED: Na2CO3(s) + 2HCl(aq) â†' 2NaCl(aq) + H2O(l) + CO2(g) Using the balanced chemical reaction from step 1, calculate the mass of sodium carbonate (Na2CO3) that would be required to make 0.75




![Odia] In the reaction, H2O2 +Na2CO3 rarrNa2O2 +CO2 +H2O,the substan Odia] In the reaction, H2O2 +Na2CO3 rarrNa2O2 +CO2 +H2O,the substan](https://static.doubtnut.com/ss/web-overlay-thumb/8303055.webp)