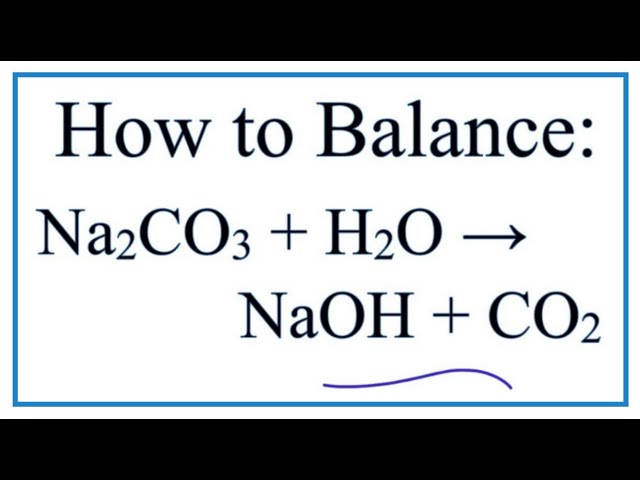
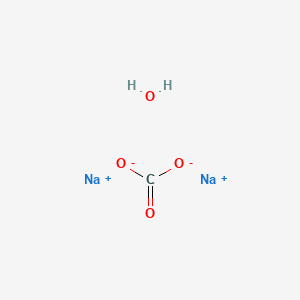
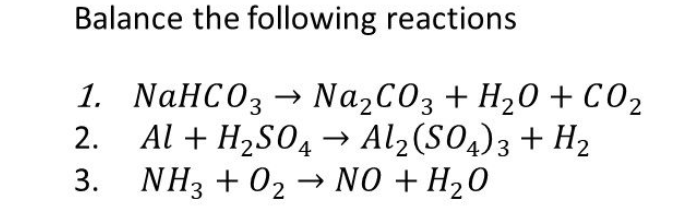How to Balance Na2CO3+H2O→NaOH+CO2 | How to Balance Na2CO3+H2O→NaOH+CO2 | By Chemistry 360 | Facebook
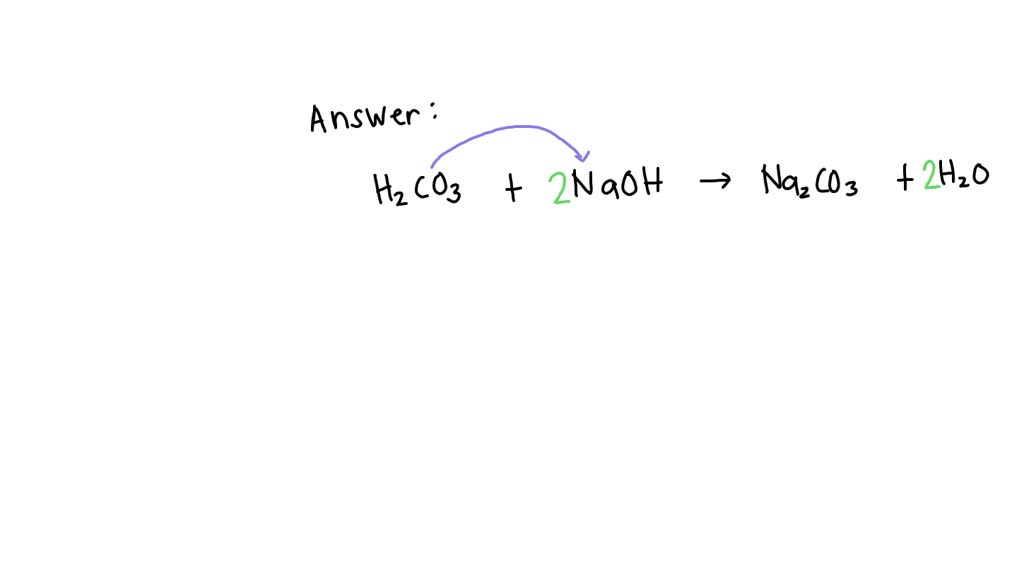
SOLVED: H2CO3 + NaOH → Na2CO3 + H2O identify its type of reaction,states of matter and its balanced equation

How many mL of 0.1 M HCl are required to react completely with 1 g mixture of Na2CO3 and NaHCO3 containing equimolar amounts of b… | Test tube, Solutions, Completed