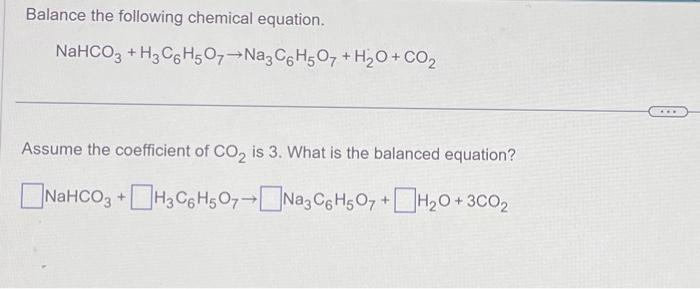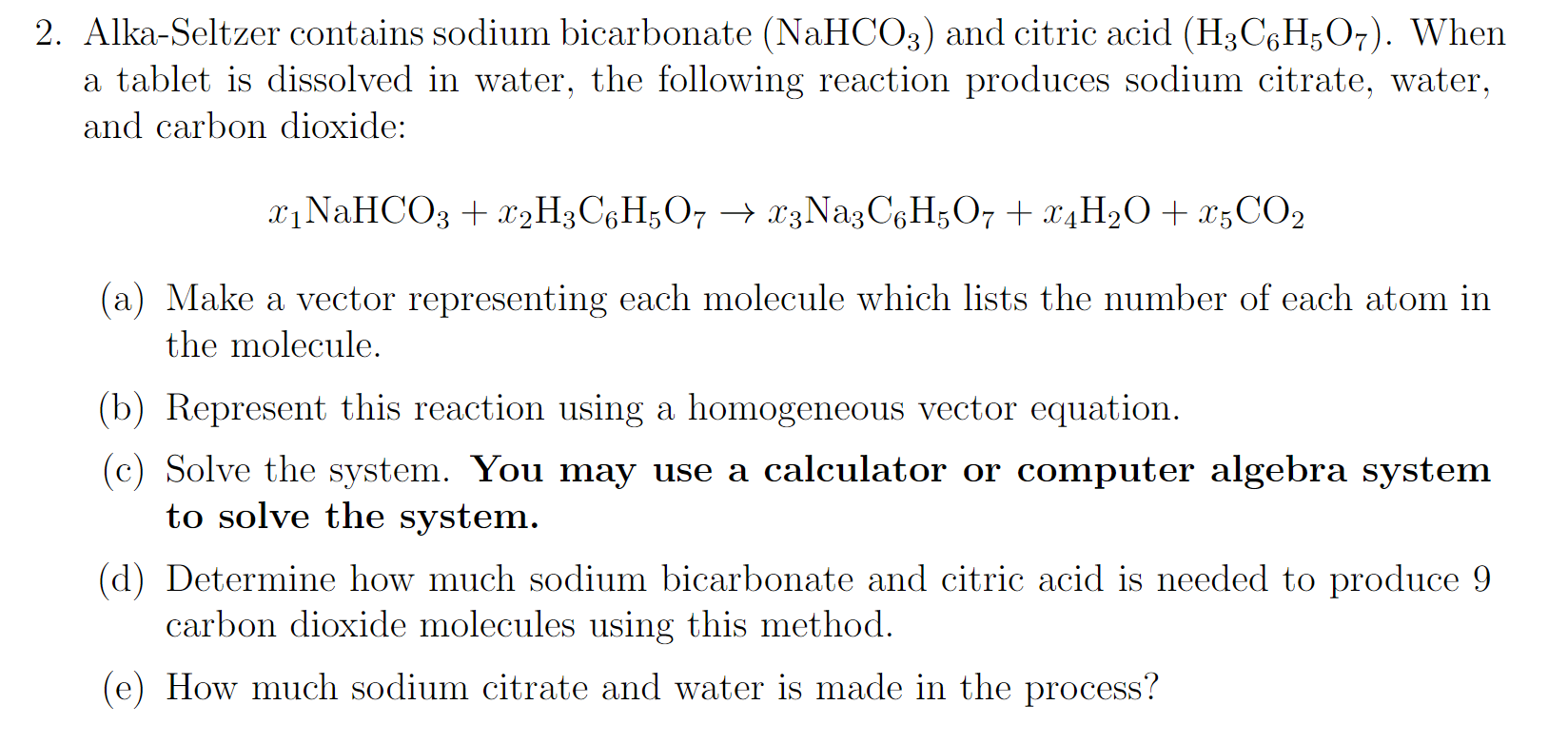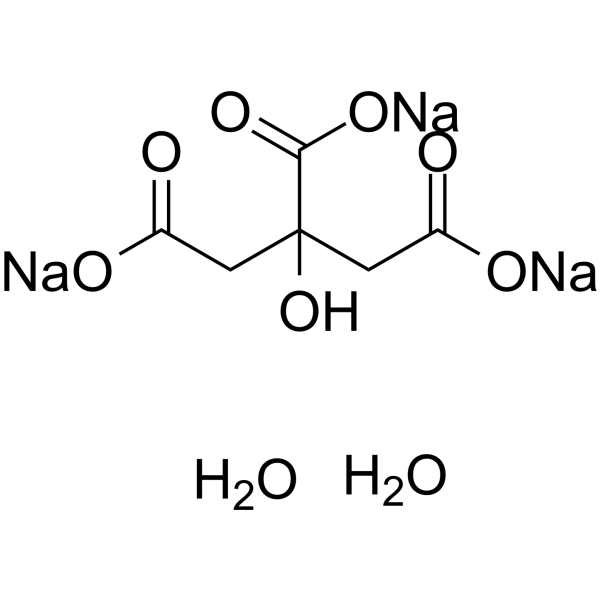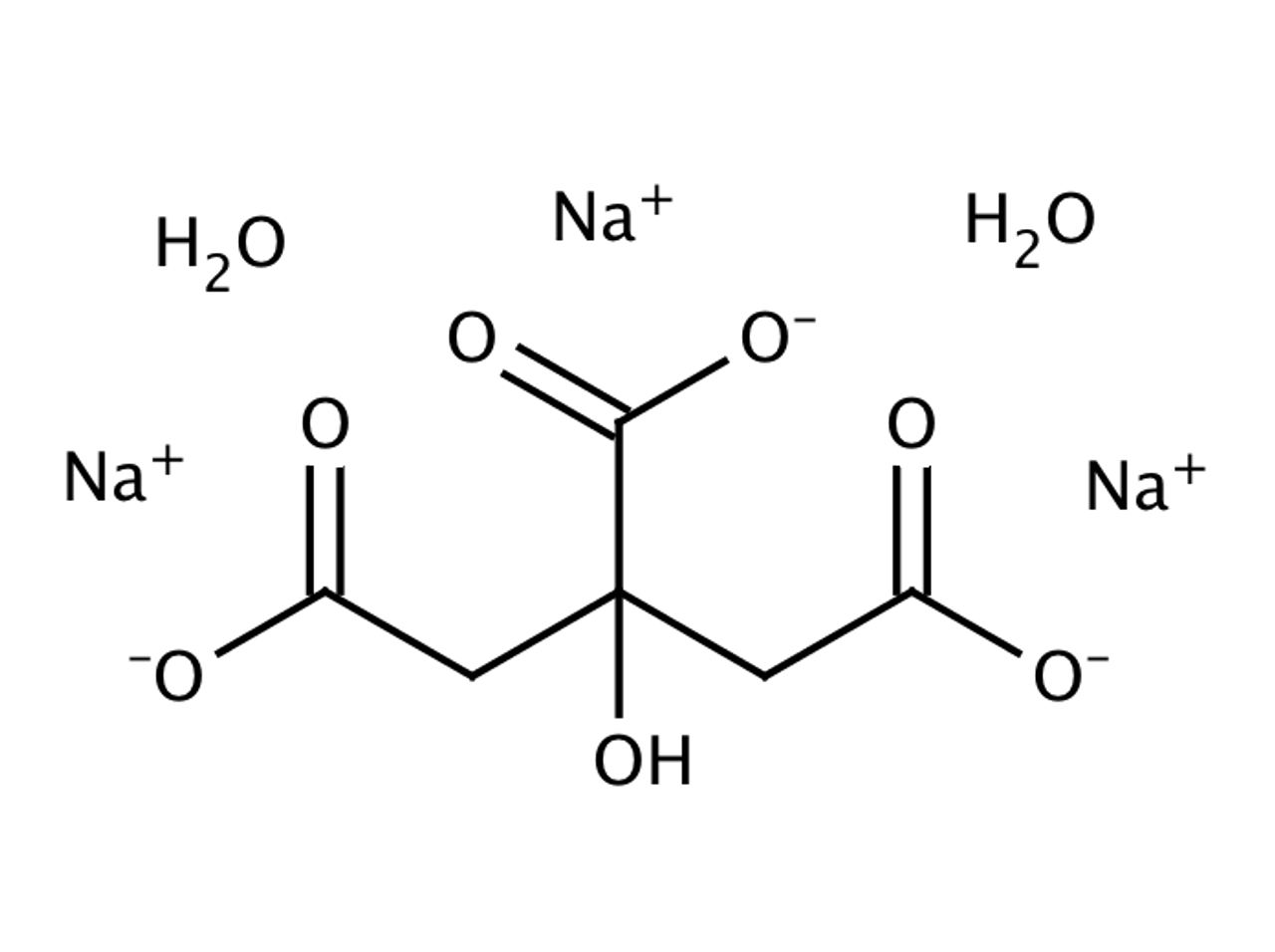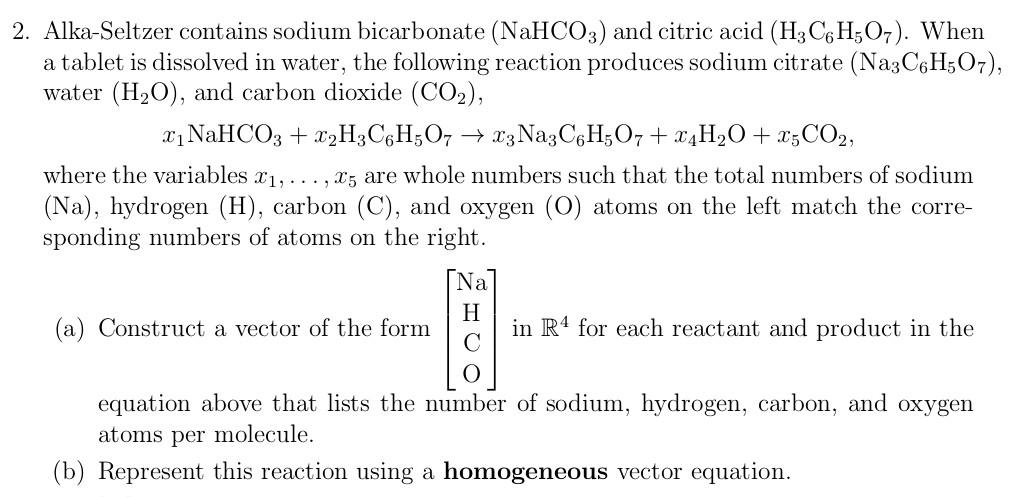
SOLVED: Aka-setter contains sodium bicarbonate (NaHCO3) and the citric acid (H3C6H5O7). When the tablet is dissolved in water, the following reaction produces sodium citrate (Na3C6H5O7), water (H2O), and carbon dioxide gas (CO2): (
![Phase diagrams for [C4mim]BF4 + Na3C6H5O7 + H2O ABS in the presence of... | Download Scientific Diagram Phase diagrams for [C4mim]BF4 + Na3C6H5O7 + H2O ABS in the presence of... | Download Scientific Diagram](https://www.researchgate.net/publication/325860685/figure/fig1/AS:961857821081639@1606336183966/Phase-diagrams-for-the-C4mimBF4-Na3C6H5O7-H2O-ABS-in-the-presence-of-amino-acids-of_Q320.jpg)
Phase diagrams for [C4mim]BF4 + Na3C6H5O7 + H2O ABS in the presence of... | Download Scientific Diagram

SOLVED: Soda fizz comes from sodium bicarbonate and citric acid (C6H8O7) reacting to make carbon dioxide, sodium citrate (Na3C6H5O7), and water. Note: the equation is balanced 3 NaHCO3(aq) + C6H8O7(aq) —> 3CO2(g) +

How to Balance H3C6H5O7 + NaHCO3 = CO2 + H2O + Na3C6H5O7 (Citric acid + Sodium bicarbonate ) - YouTube

Reduction of HAuCl4 with sodium citrate in water: the sizes of AuNPs... | Download Scientific Diagram

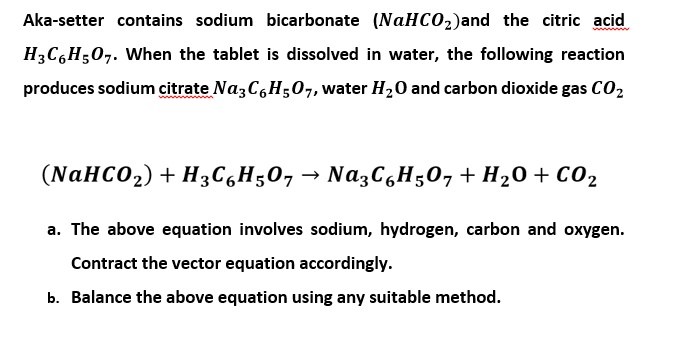
SOLVED: Alka Seltzer contains sodium bicarbonate (NaHCO3) and citric acid (H3C6H5O7). When the tablet is dissolved in water, the following reaction produces sodium citrate, water, and carbon dioxide (gas). Balance the chemical

SOLVED: The Alka Seltzer reaction involves citric acid and sodium bicarbonate (baking soda) according to the following unbalanced equation: H3C6H5O7 + NaHCO3 -> Na3C6H5O7 + H2O + CO2. Starting from 1.3 g
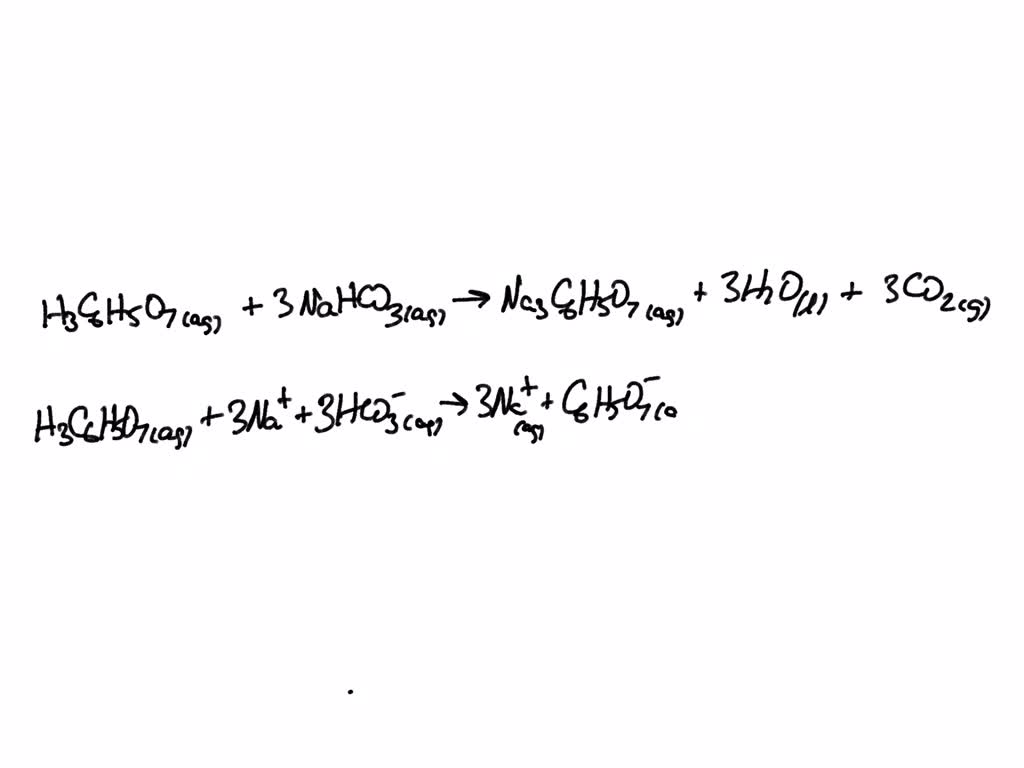
SOLVED: Write the balanced net ionic equation for the reaction between aqueous solutions of citric acid and sodium bicarbonate. H3C6H5O7 (aq) + NaHCO3 (aq) = Na3C6H5O7 (aq) + CO2 (g) + H2O (l)
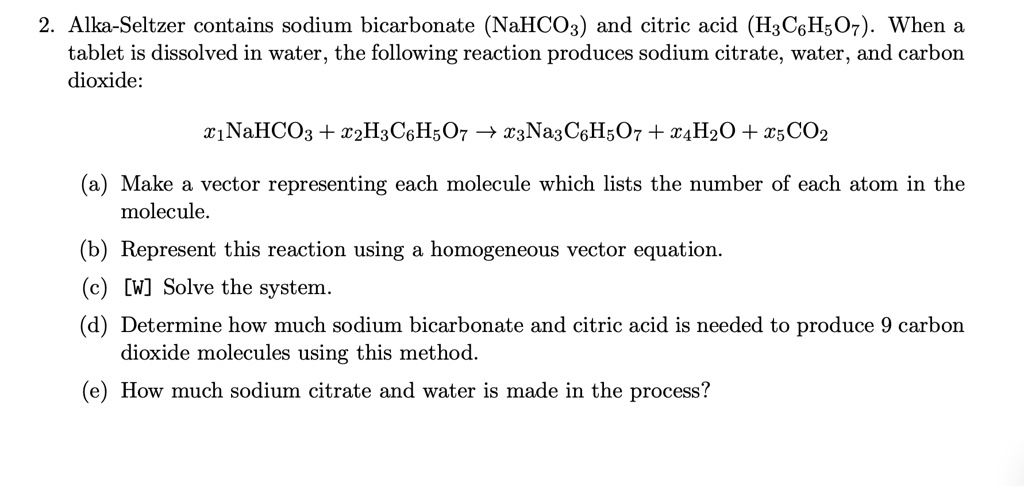
SOLVED: Alka-Seltzer contains sodium bicarbonate (NaHCO3) and citric acid (H3C6H5O7). When the tablet is dissolved in water, the following reaction produces sodium citrate, water, and carbon dioxide: 2NaHCO3 + H3C6H5O7 â†' Na2C6H5O7 +
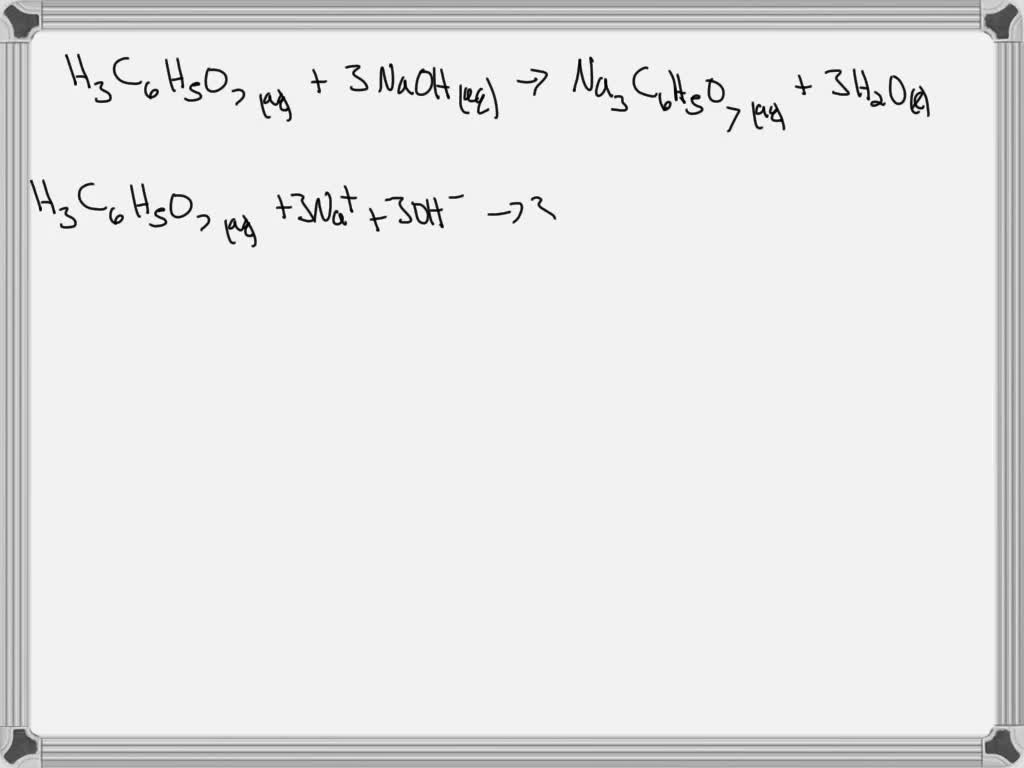
SOLVED: H3C6H5O7(aq) + 3 NaOH(aq) = Na3C6H5O7(aq) + 3 H2O(l) write complete ionic and net-ionic equations
![Phase diagrams for [C4mim]BF4 + Na3C6H5O7 + H2O ABS in the presence of... | Download Scientific Diagram Phase diagrams for [C4mim]BF4 + Na3C6H5O7 + H2O ABS in the presence of... | Download Scientific Diagram](https://www.researchgate.net/publication/325860685/figure/fig4/AS:961857825292304@1606336184782/Phase-diagrams-for-C4mimBF4-Na3C6H5O7-H2O-ABS-in-the-presence-of-different-mass.png)