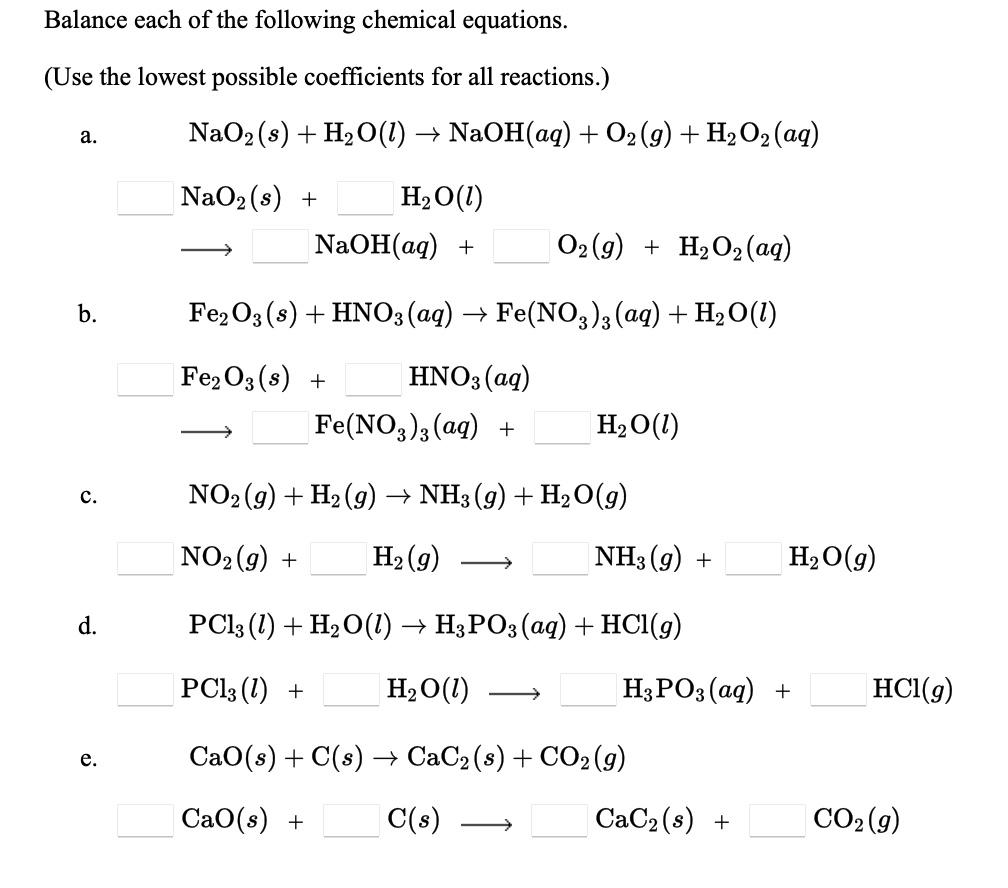
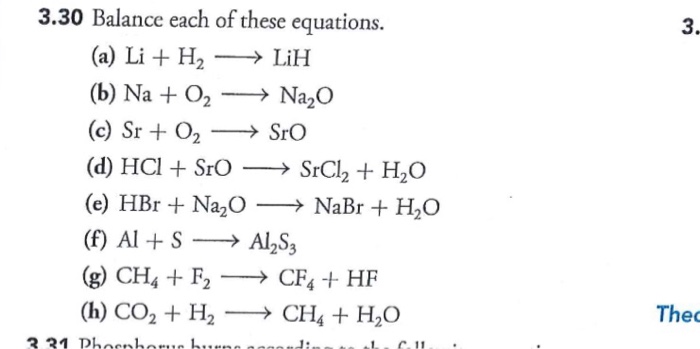
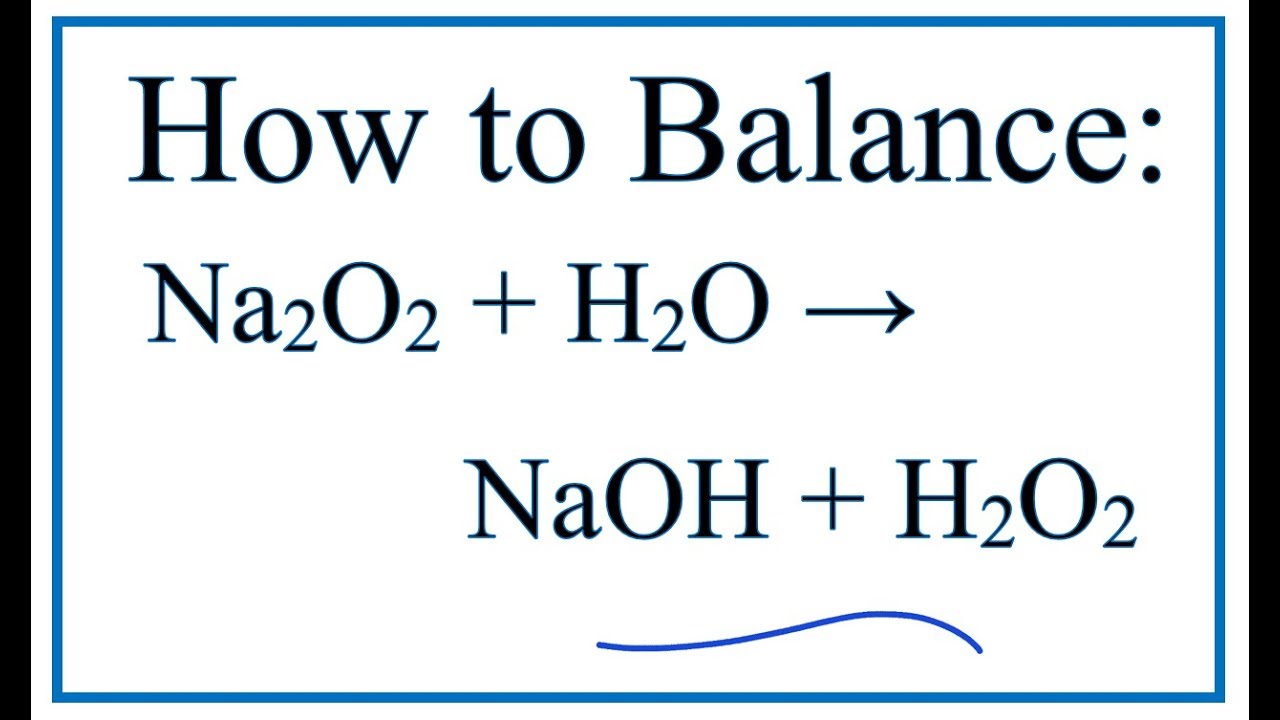
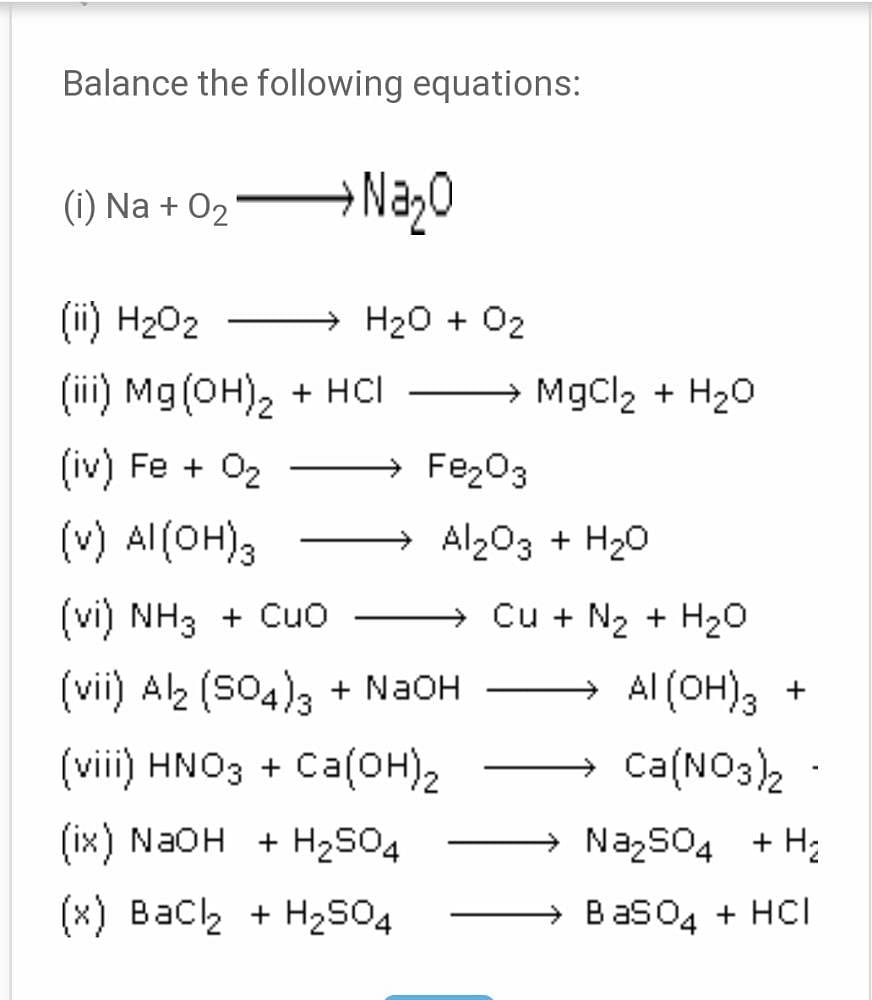
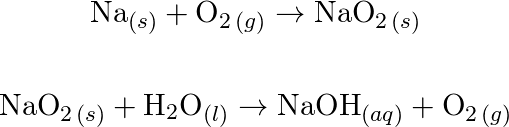Highly Homogeneous Sodium Superoxide Growth in Na–O2 Batteries Enabled by a Hybrid Electrolyte | ACS Energy Letters

A Highly Stable All‐Solid‐State Na–O2/H2O Battery with Low Overpotential Based on Sodium Hydroxide - Jiang - 2022 - Advanced Functional Materials - Wiley Online Library
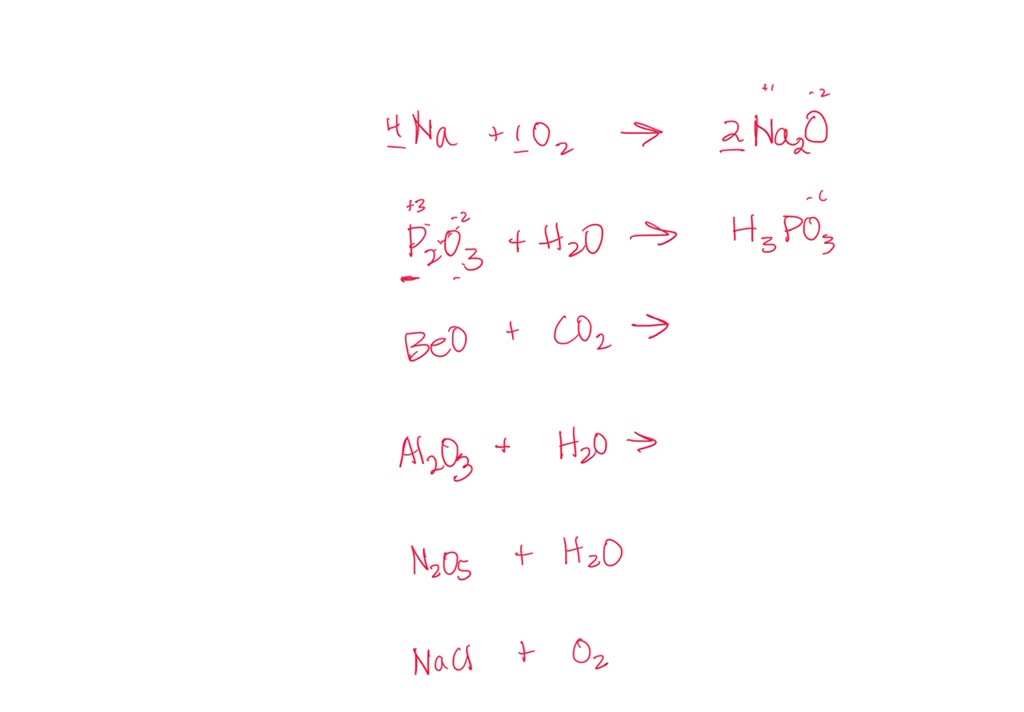
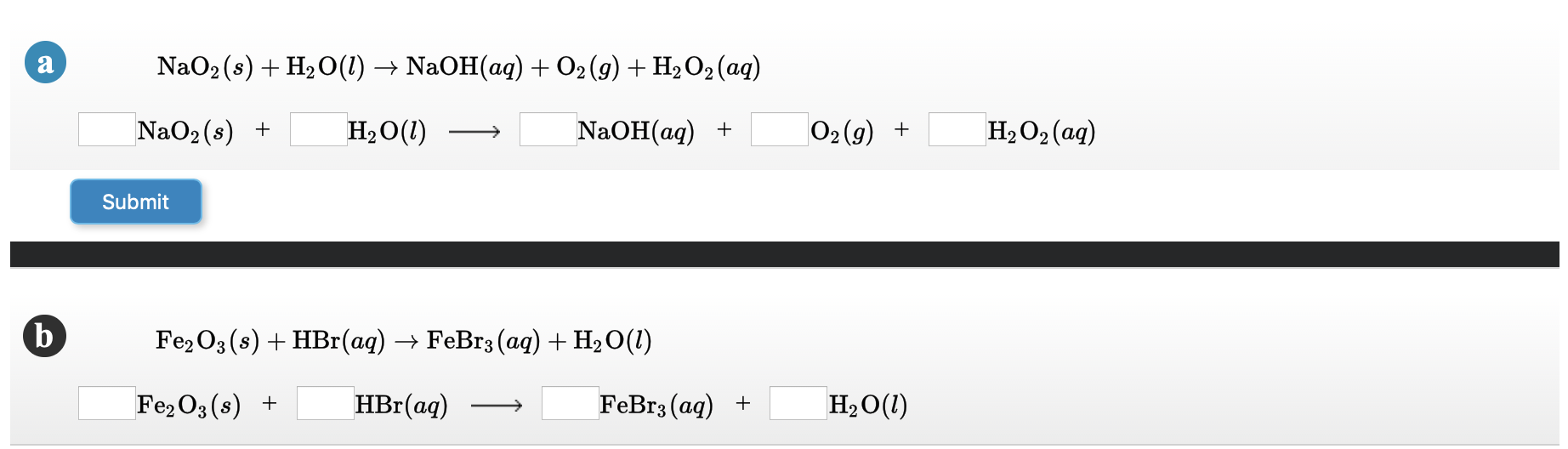
SOLVED: Identify the products of these synthesis reactions and balance the reactions: 6. Na + O2 → 7. P2O3 + H2O → 8. BeO + CO2 → 9. Al2O3 + H2O →

Table 2 from Homogeneous Oxidation of Volatile Nitrogen (NH3, HCN) to Nitrogen Oxides: A Modeling Study of the Effect of Alkali Vapors | Semantic Scholar

Balance the following chemical equation. a) Na + O2 → Na2O b) Ca + N2 + Ca N2 c) N2 + H2 →NH d) CaCO3 +HCl - Brainly.in

HELP Combination (or synthesis) – two or more elements combine to form a compound. Na + O2 Na2O - brainly.com