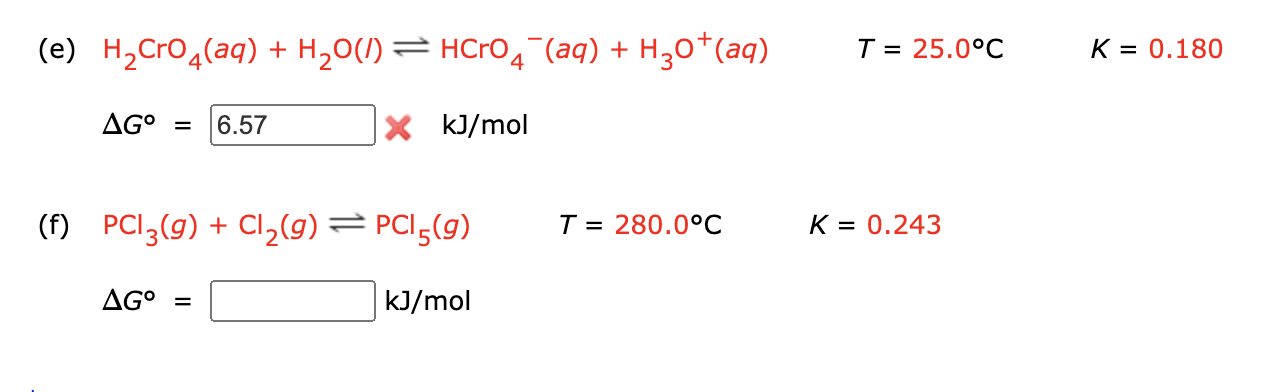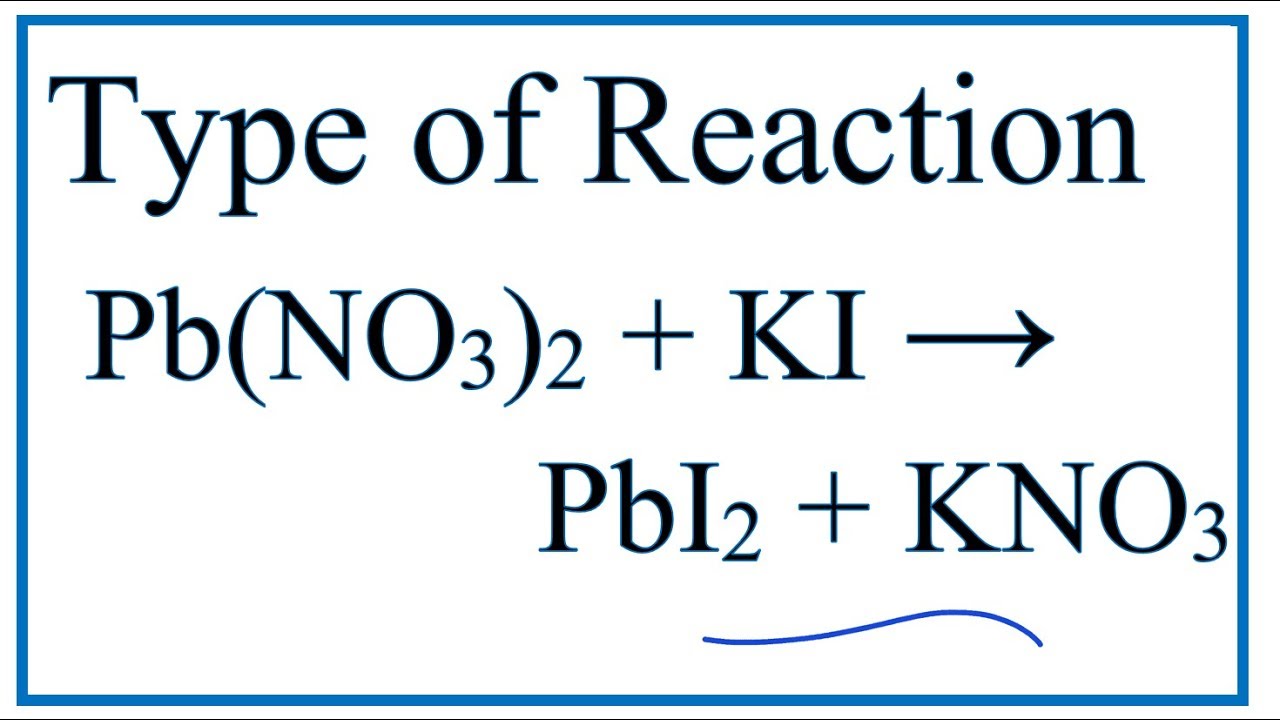Improvement of Colloidal Characteristics in a Precursor Solution by a PbI2-(DMSO)2 Complex for Efficient Nonstoichiometrically Prepared CsPbI2.8Br0.2 Perovskite Solar Cells | ACS Applied Materials & Interfaces

Orientation-Controlled (h0l) PbI2 Crystallites Using a Novel Pb–Precursor for Facile and Quick Sequential MAPbI3 Perovskite Deposition | ACS Omega

Few-Layer PbI2 Nanoparticle: A 2D Semiconductor with Lateral Quantum Confinement | The Journal of Physical Chemistry Letters

Perovskite Solar Cell Stability in Humid Air: Partially Reversible Phase Transitions in the PbI2-CH3NH3I-H2O System - Advances in Engineering

SOLVED: 15.8 mol H2O 10^16 CuCl2 PbI2 (2H2O) 9d CH4 7u4s TKsC 9.60 mol CO2 20 °C 64 x 10^25 kg C96yne 74010, '.` e Ua 'decoxide contains 'tetraphosphorus = L X
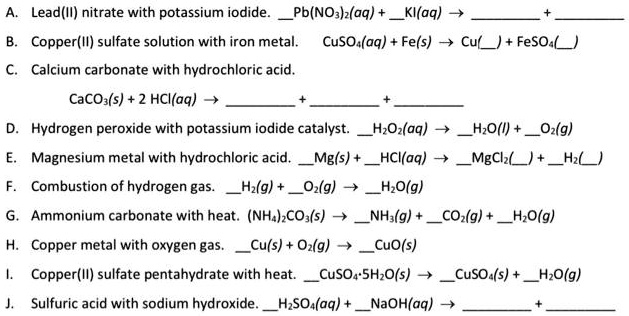
SOLVED: A. Lead(II) nitrate with potassium iodide. Pb(NO3)2(aq) + 2KI(aq) -> PbI2(s) + 2KNO3(aq) B. Copper(II) sulfate solution with iron metal. CuSO4(aq) + Fe(s) -> Cu(s) + FeSO4(aq) C. Calcium carbonate with

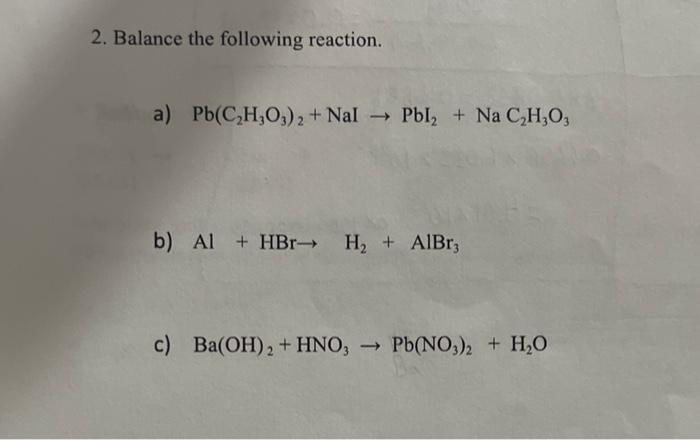
SOLVED: Texts: 1. Balance the following chemical equations. a. Na3PO4 (aq) + Al(NO3)3(aq) -> NaNO3(aq) + AlPO4(s) b. Pb(NO3)2 (aq) + 2 KI (aq) -> PbI2(s) + 2 KNO3(aq) c. Fe (s) +
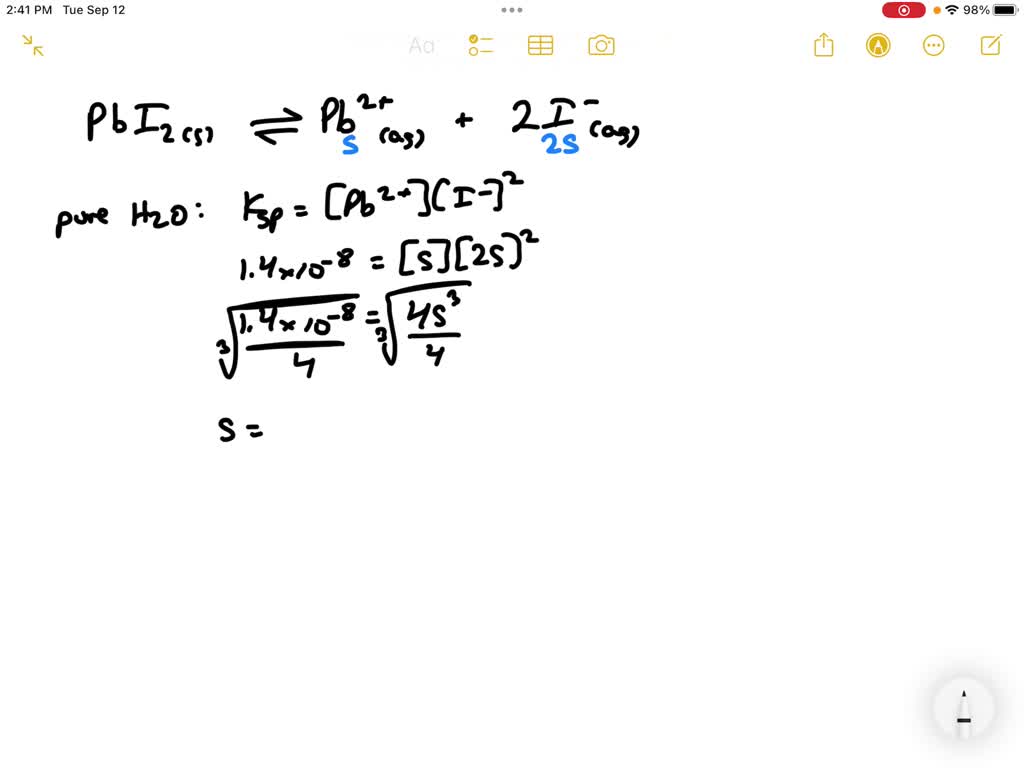
SOLVED: 2. Calculate the molar solubility of PbI under two different conditions when PbI2 is added to pure H2O and when it is added to a 1.5 M solution of CaI2. The

Controlling PbI2 Stoichiometry during Synthesis to Improve the Performance of Perovskite Photovoltaics | Chemistry of Materials

a XRD of PbI2 and an as-converted perovskite film. Peaks referring to... | Download Scientific Diagram