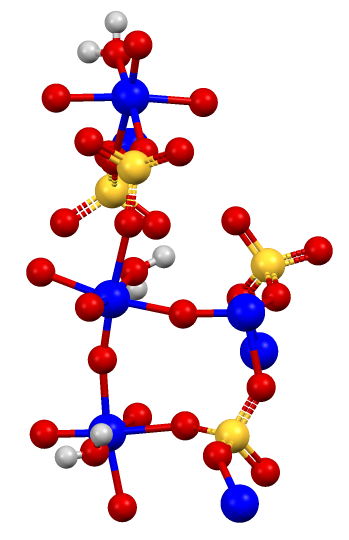
The Effect of SO2 and H2O on the Interaction Between Pt and TiO2(P-25) During Catalytic CO Oxidation | Catalysis Letters

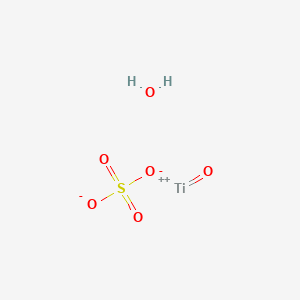
Nanomaterials | Free Full-Text | Preparation of Hydrated TiO2 Particles by Hydrothermal Hydrolysis of Mg/Al-Bearing TiOSO4 Solution

Processes | Free Full-Text | Mechanism, Thermodynamics and Kinetics of Rutile Leaching Process by Sulfuric Acid Reactions
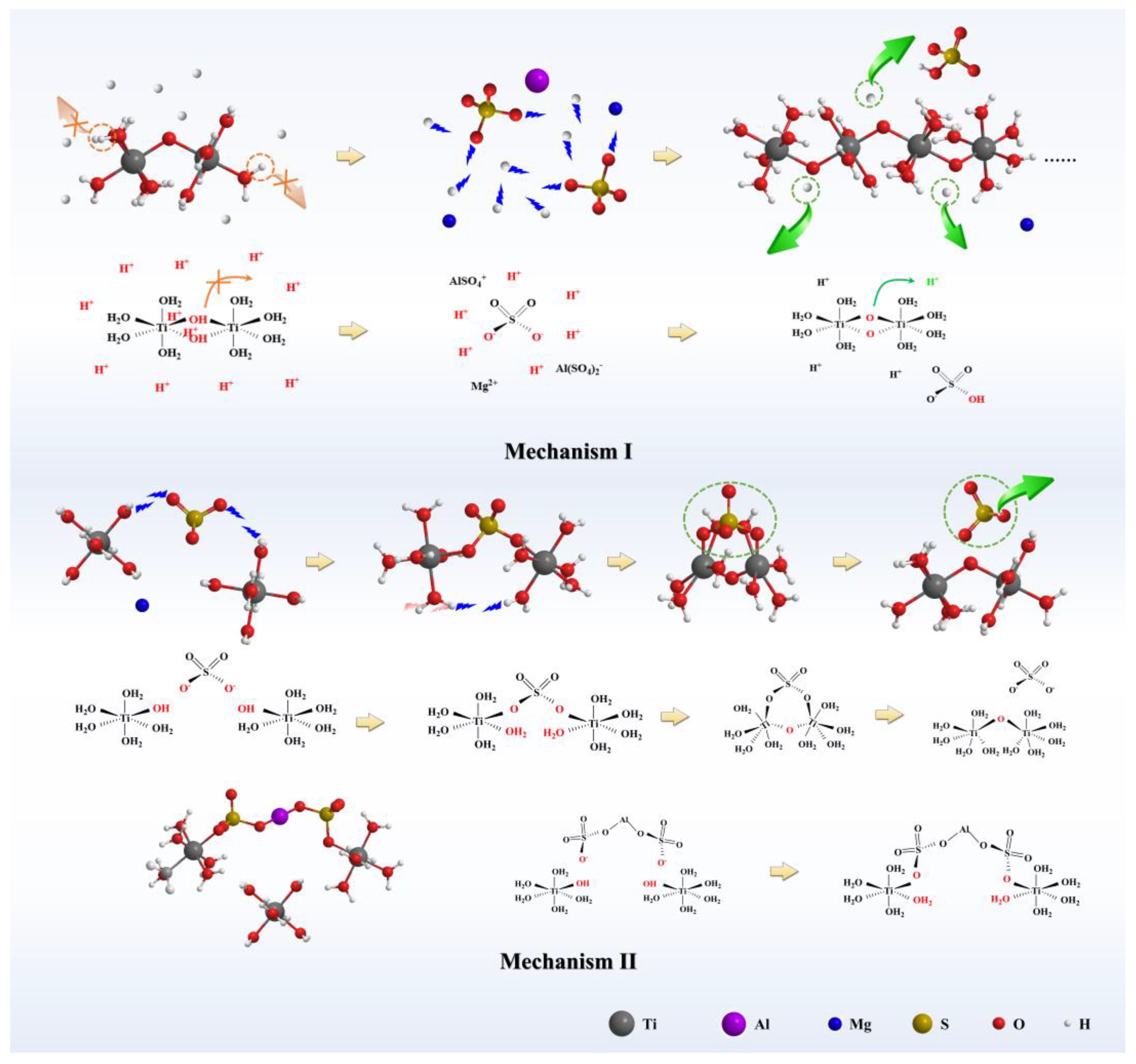
Nanomaterials | Free Full-Text | Preparation of Hydrated TiO2 Particles by Hydrothermal Hydrolysis of Mg/Al-Bearing TiOSO4 Solution

PDF) Effect of TiOSO 4 hydrothermal hydrolysis conditions on TiO 2 morphology and gas-phase oxidative activity | Alexander Vorontsov - Academia.edu
Synthesis and structural characterisation of solid titanium(IV) phosphate materials by means of X-ray absorption and NMR spectro

Thermodynamic data for chemical transformations in the TiO2-SO3-H2O system. | Download Scientific Diagram

Clean, low-cost, and efficient nano-TiO2 composite photocatalyst: Preparation and performance in TiOSO4 hydrolysis system - ScienceDirect
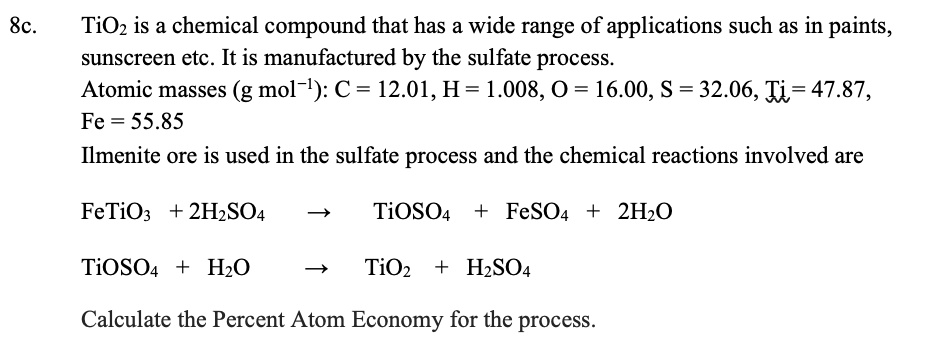
SOLVED: TiO2 is a chemical compound that has a wide range of applications such as in paints, sunscreen, etc. It is manufactured by the sulfate process. Atomic masses (g mol-1): C =

SOLVED: The flowsheet below represents the process for the production of TiO2. Sorel Slag Scrap Iron (Pure Fe) HSO4 67% by weight H2SO4 Digester Evaporator FeSO4 Air TiO2 Pure O2 Rotary Kiln

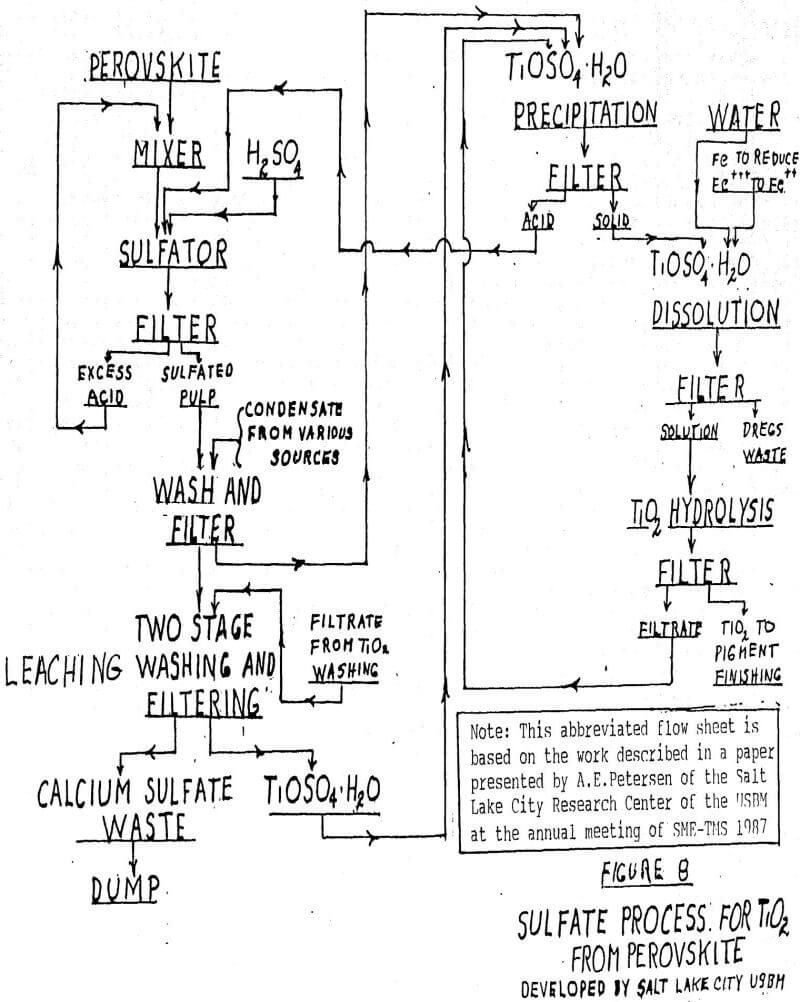
Cristal History 1988 – The company incorporated 1991 – The first pigment was produced 1992 – Fully operated with 4 grades at 48,000 tpa 2000 – CRISTAL. - ppt download

Characterization of Chemical Speciation of Titanyl Sulfate Solutions for Production of Titanium Dioxide Precipitates | Inorganic Chemistry

SEM images of TiO2 products at various H2O/ TiOSO4 volume ratio of a... | Download Scientific Diagram

Supramolecular self-assembly synthesis of ordered mesoporous TiO2 from industrial TiOSO4 solution and its photocatalytic activities - ScienceDirect





![PDF] Influence of hydrolysis in sulfate process on titania pigment producing | Semantic Scholar PDF] Influence of hydrolysis in sulfate process on titania pigment producing | Semantic Scholar](https://d3i71xaburhd42.cloudfront.net/f51fdcb711813023bae94e22833f7e56d341cbd6/3-Table2-1.png)